2.1.3 Nucleic acid structure
2.1.3 Nucleotides and nucleic acids (a-d)
Nucleotide and nucleic acid structure - Notes
2.1.3a: the structure of a nucleotide as the monomer from which nucleic acids are made
Draw, and label, the basic structure of a nucleotide. (F)
Draw a nucleotide showing the structure of the pentose sugar and where the phosphate group and nitrogenous base attach using the standard system for numbering the carbons in the sugar.
State the two main types of nucleic acid. (F)
Draw a table to show the similarities and differences between the nucleotides of DNA and RNA. (F)
Draw the structures of ribose and deoxyribose and identify the difference between the two pentose sugars.
List the full names of the 5 possible nitrogenous bases in nucleic acids.
Name the two types of nitrogenous base and state which bases belong to which type.
Outline the structure of the two types of nitrogenous base.
2.1.3b: the synthesis and breakdown of polynucleotides by the formation and breakage of phosphodiester bonds
Define the terms “monomer”, “polymer”, “nucleic acid”, “polynucleotide”, “nucleotide”, and “phosphodiester bond”. (F)
Draw and label a diagram to show how nucleotides can link together to form polynucleotides (including the production of water). (F)
State the name of the reaction that joins nucleotides to other nucleotides and the name of the reaction that breaks phosphodiester bonds. (F)
2.1.3c: the structure of ADP and ATP as phosphorylated nucleotides
State 3 main types of activity for which cells require energy.
Draw and label a diagram of ATP and ADP. (F)
List 2 similarities and 2 differences between the structure of ATP and DNA and RNA nucleotides.
Draw a reaction to show how energy is released from ATP to provide energy for cellular activities. (F)
Draw a diagram to show the interconversion of ATP and ADP, the names of the types of reactions involved, where energy is released and the role of respiration.
State 5 properties of ATP and explain why each makes it ideally suited to function as an energy transfer molecule. (F)
Define the term “phosphorylation”.
2.1.3d: (i) the structure of DNA (deoxyribonucleic acid). (ii) practical investigations into the purification of DNA by precipitation
Draw and label a diagram of the structure of DNA. (F)
Define the terms “complementary base pairing”, “sugar-phosphate backbone”, “antiparallel”, “double helix”, and “strand” in relation to DNA. (F)
State the complementary base pairing rules, name the bond that holds them together, and state the number of bonds that hold each pair together. (F)
Explain why a DNA molecule has equal amounts of adenine and thymine and equal amounts of cytosine and guanine.
Describe how purines and pyrimidines are arranged in the complementary base pairing rules.
Describe the significance of the double stranded, complementary base paired nature of DNA for its function.
Describe the significance of the sequence of bases in a DNA strand for its function.
Describe, and explain the importance of the steps in the isolation and purification of DNA by precipitation.
Name the people involved in the discovery of the structure of DNA and describe the evidence used to work out this structure. (S+C)
Extracting DNA
In a school laboratory DNA is often extracted from strawberries or kiwi fruit. The cells of the fruit need to be broken and the membranes broken down to release the DNA. The DNA then needs to be precipitated so that it can be seen.
You need to be familiar with the steps in the method and what they do:
Cut up your sample and grind it with a pestle and mortar (sometimes with a bit of sand)
This rips apart cell walls so that the contents can be released.
This also breaks the tissue into very small pieces increasing the surface area that is exposed to the chemicals in future steps
Add some detergent to sample in the mortar
This breaks down the cell surface membrane and the nuclear envelope (phospholipids can now dissolve in the solution thereby disrupting membrane structure). The DNA is released into the solution
Add salt (NaCl) to the sample in the mortar
The charges on the phosphates of the sugar-phosphate backbone are neutralised (i.e. they bind ionically to the Na+ ions). This breaks the hydrogen bonds between the DNA and water molecules and makes the DNA less soluble
Add protease enzyme (some plant material naturally has high levels of protease in it already)
This breaks down the proteins associated with DNA (histones)
Filter the sample through muslin into a test tube
This removes the solid plant material so that the DNA precipitate will be easier to see
Ideally a low temperature is maintained throughout
This reduces the rate of enzyme-controlled reactions that hydrolyse DNA.
Add a layer of ice-cold ethanol on top of the sample
DNA is soluble in water but insoluble in ethanol. The DNA precipitates out of solution at the interface between the water and ethanol layers. (If the ethanol is ice-cold then DNA is even less soluble in it and more precipitation will occur)
Look for white strands at the interface between the ethanol and the water layers
DNA appears as white strands when it precipitates
Nucleotides
Nucleotides are the building blocks (the monomers) of polynucleotides such as DNA and RNA (the polymers). Nucleotides are important in cells in their own right too – particularly as ADP and ATP which shuttle energy around the cell to power the cell’s actions.
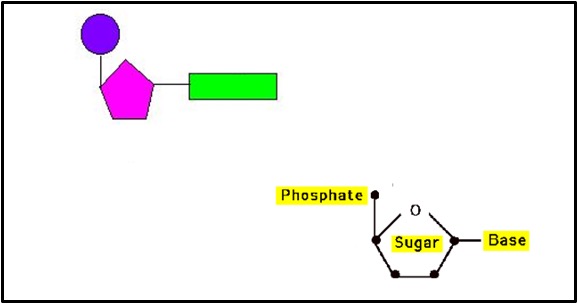
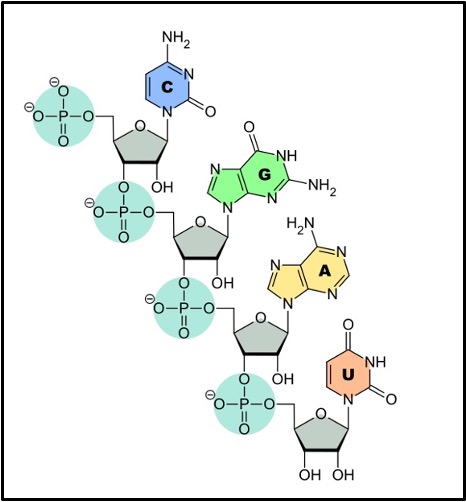
A nucleotide (shown in the box above) is made of 3 components joined together by condensation reactions. These components are:
A pentose sugar (deoxyribose in DNA nucleotides or ribose in RNA nucleotides)
A phosphate group joined to the carbon 5 of the pentose sugar
A nitrogenous base (A, T, C or G in DNA nucleotides or A, U, C or G in RNA nucleotides) joined to the carbon 1 of the pentose sugar
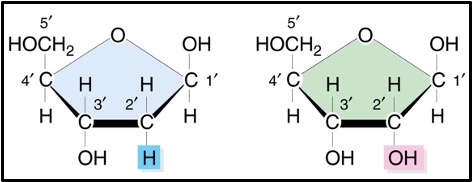
Ribose is a standard pentose sugar and has the formula C5H10O5.
Deoxyribose has one fewer oxygen atoms than ribose and so its formula is C5H10O4. There is an hydroxyl group on carbon 2 in ribose but just a hydrogen in deoxyribose – see the box below)
There are two types of nitrogenous base:
Pyrimidines are single ring structures (top row of the box below). Cytosine (C), Thymine (T) and Uracil (U) are all pyrimidines.
Purines are double ring structures (bottom row of the box below). Adenine (A) and Guanine (G) are both purines.
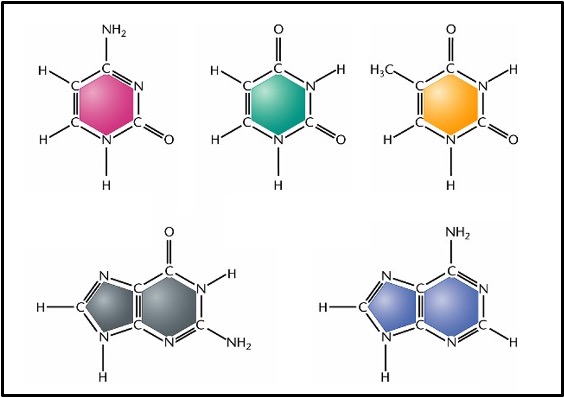
You don’t need to know the detailed structure of the nitrogenous bases but you do need to know which ones are purines, which are pyrimidines and that purines have two rings in their structure whereas pyrimidines only have one.
Polynucleotides
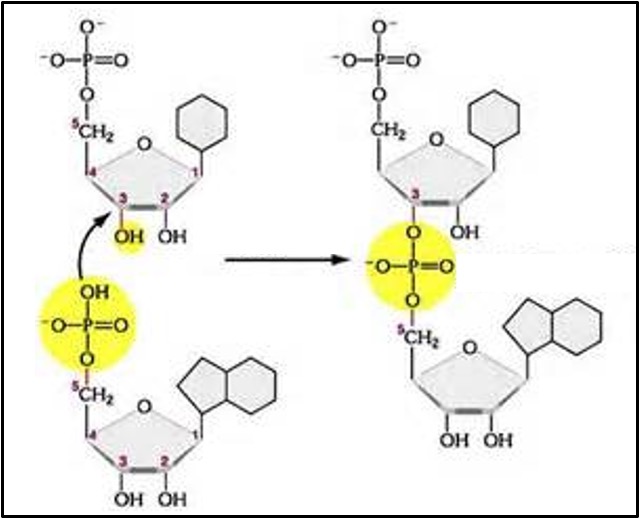
Nucleotides join together in condensation reactions to form dinucleotides, trinucleotides and eventually polynucleotides. The bond that joins nucleotides together is called a phosphodiester bond. It joins the phosphate group on one nucleotide to carbon 3 of another nucleotide (see the box below).
Both DNA and RNA are polynucleotides. DNA stands for deoxyribonucleic acid and RNA stands for ribonucleic acid. So, both DNA and RNA are types of nucleic acid.
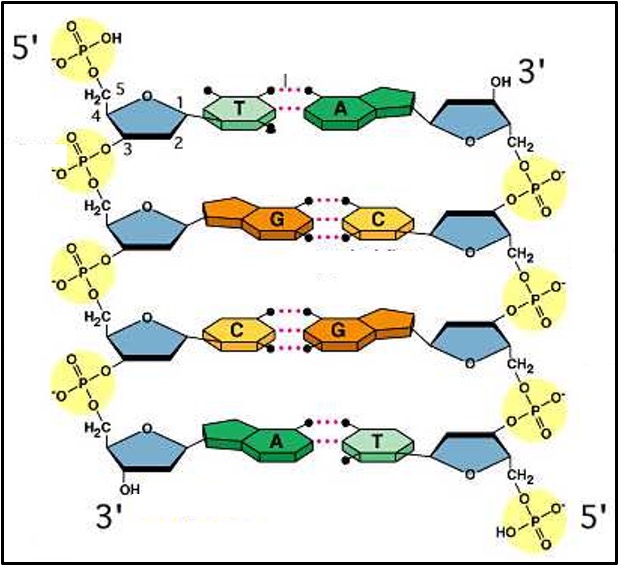
Structure of DNA
DNA is double stranded (except in some viruses). This means it has two polynucleotide strands joined together. Be careful to use the terms ‘DNA molecule’ and ‘DNA strand’ correctly.
Each DNA strand has a sugar-phosphate backbone with the nitrogenous bases joined by hydrogen bonds to the nitrogenous bases of the other strand.
The two DNA strands are parallel but the nucleotides in one strand are the other way up compared to the ones in the other strand (see the 3’ and 5’ labelling of the carbons in the pentose sugars in the diagram). To describe this the two strands in DNA are called ‘antiparallel’.
The two strands in DNA twist around each other forming a ‘double helix’.
The nitrogenous bases from one strand cannot join to any nitrogenous base on the other strand. Each base can only pair with one other base.
The pairings, called ‘complementary base pairs’ are:
Adenine (A) joins to Thymine (T) with two hydrogen bonds
Cytosine (C) joins to Guanine (G) with three hydrogen bonds
Each pair uses one purine and one pyrimidine. This means that there are always 3 rings structures (one from the pyrimidine and two from the purine) between the two sugar-phosphate backbones.
The base pairing rules mean that a DNA molecule will always have the same number of cytosines as guanines and the same number of adenines as thymines (because where there’s one of them there is always the other on the opposite strand).
Also, half of the bases in DNA are always purines and half are always pyrimidines because where there is one on one strand there is always the other on the other strand.
How structure links to function in DNA
The fact that DNA is double stranded and that there are complementary base pairing rules means that DNA replication is possible. The two strands can separate, and each strand can act as a template to build the missing strand. This results in two identical DNA molecules that are also identical to the original DNA molecule.
The fact that a molecule of DNA can have nitrogenous bases in any order means that it can carry the genetic code. The sequence of bases in DNA codes for the sequence of amino acids in proteins.
Watson and Crick’s original scientific paper on the structure of DNA

Structure of RNA
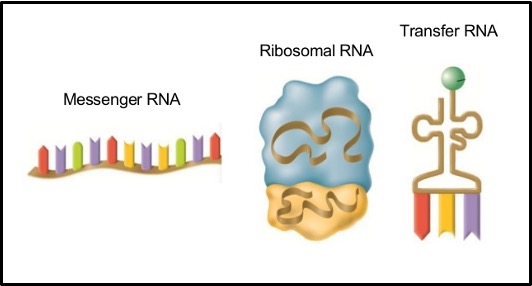
RNA, ribonucleic acid, is single stranded (except in some viruses).
There are 3 main types of RNA, all of which are involved in protein synthesis. They are:
Messenger RNA (mRNA)
This is a molecule that carries a copy of the genetic code for making a single protein from the nucleus to the ribosomes.
Ribosomal RNA (rRNA)
Ribosomes are made up of this type of RNA and proteins.
Transfer RNA (tRNA)
tRNA is single stranded but it doubles back on itself so some parts of the strand complementary base pair with other parts of the strand.
tRNAs have an amino acid attached at one end which the carry to the ribosome for protein synthesis.
A particular set of 3 bases in the RNA strand that makes up tRNA is called the anticodon and this sequence determines which of the 20 amino acids that particular tRNA molecule carries.
RNA uses ribose as its pentose sugar.
The nitrogenous bases in RNA are Cytosine (C), Guanine (G), Adenine (A) and uracil (U). Where there is complementary base pairing in RNA (in tRNA and between mRNA and tRNA in protein synthesis) the complementary base pairs are:
Adenine with Uracil
Cytosine with Guanine
ATP
Cells need energy to do their jobs (e.g. synthesis of molecules, transport of molecules, movement). Some jobs that require a source of chemical energy are:
Active transport
Moving chromosomes around in mitosis/meiosis
Moving vesicles (e.g. in exocytosis)
Metabolic reactions (e.g. protein synthesis)
Moving cells or whole organisms (e.g. muscle contraction, movement of flagella)
In humans, most cells receive their chemical energy as glucose from the blood. However, glucose itself would be a very poor source of energy for individual jobs within a cell as too much energy would be wasted (there’s a lot of energy in glucose compared to the amount of energy each little job needs) and it takes a lot of different enzymes to fully break down glucose.
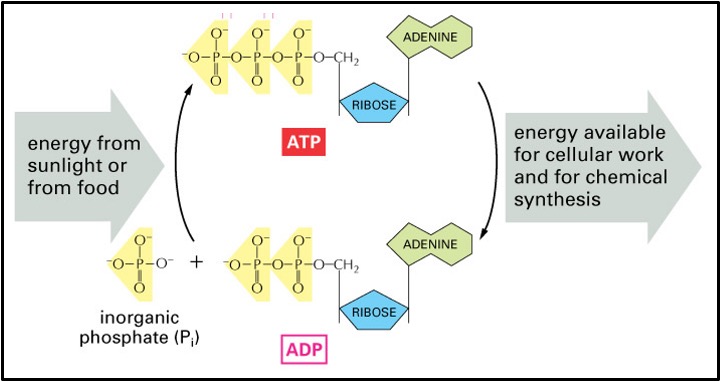
Instead, glucose is used in aerobic respiration where the energy in glucose is transferred to a molecule called ATP (Adenosine triphosphate). In respiration the energy from glucose is used to add a phosphate onto ADP (adenosine diphosphate) to form ATP. When the ATP is used in the cell to do a job the third phosphate breaks off and ADP is reformed. Overall, this reaction releases energy which is used to do the job in the cell. The ADP and the phosphate (now called an inorganic phosphate ion (Pi) return to where aerobic respiration is occurring to be turned back into ATP. In this way ATP and ADP+Pi keep cycling round the cell transferring the energy released in respiration to where it is needed in the cell.
The addition of a phosphate group to a molecule is called phosphorylation and the removal of one is called dephosphorylation.
It takes energy to break bonds and energy is released when bonds form. Only a small amount of energy is needed to break off the phosphate but lots of energy is released when that phosphate forms bonds (about 30.6KJmol-1)
ATP is an excellent molecule for shuttling energy around a cell because:
It is small and so can move easily and quickly, by diffusion, around the cell.
It is water soluble and so doesn’t leave the cell (it can’t pass through a phospholipid bilayer). It can also be involved in reactions in an aqueous environment.
It releases energy in a single step reaction and so energy can be released quickly with the presence of a single enzyme (an ATPase).
It is easily regenerated so only a small quantity of ATP is need in any one cell as, once used, it can quickly be reformed so long as energy is supplied from respiration.
The reaction involved when the third phosphate breaks off releases just the right amount of energy – it’s large enough to be useful without requiring too many ATP molecules but not too large that the energy needed for a job cannot be very well-matched resulting in a lot of energy being wasted as heat.