Plant 14 🪴😭
PARENT MATERIAL - ROCKS AND MINERALS
Rocks
Igneous -
Solidified Magma
Granite, gabbro
Composed of primary mineral
Sedimentary -
Weathered minerals
sediment in lakes and oceans
Cemented into rock
Sandstone, shale
Composed of secondary minerals
Metamorphic -
Once igneous and sedimentary rocks
high temperature and pressure change form and structure
Gneiss, schist, slate
Primary Mineral
Formed during original crystallization of igneous rock
Quartz, feldspar, mica, hornblende, biotite
Secondary Mineral -
Weathered primary minerals
WEATHERING PROCESSES
Weathering Defined -
physical & chemical change occurring in rocks & minerals leading to disintegration &/or decomposition:
Erosion Defined -
The removal &/or transport of minerals from rock formation, as a result of environmental factors ex. Water, ice, wind, etc
Physical (weathering) -
Disintegration into smaller particles
Without much change to chemical composition
Chemical (weathering) -
Decomposition of primary minerals
Can form secondary through recrystallization
Can lead to disintegration
PHYSICAL WEATHERING
Causes - climate
Temperature fluctuations
exfoliation - peeling of layers from parent mass
Wind
Water, ice
Cryofracturing
CHEMICAL WEATHERING AGENTS
Carbonation-
CO2 combines with water
From air or Biota
Forms carbonic acid (HCO3)
Dissolves Rock
Oxidation-
Dependant on Parent Material
Oxygen combines with minerals in rock
Form Iron Oxides (FeO)
Oxidation weakens rocks - causes disintegration
Hydrolysis
Water splits into H+ (acidic) or OH- (corrosive) ions
Replaces other mineral ions in rock
Forms new solutions
Hydration
Bonding of water and mineral
Rocks swells causing disintegration
Forms new mineral compound
Both related to Climate and Topography
MOVEMENT OF WEATHERED PARENT MATERIAL
Alluvium/alluvial - material moved by water streams
Marine - under oceans
Lacustrine - under fresh water lakes
Glacial Till - Material collected and moved by glaciers
Eolian - material moved by wind
Colluvium - material moved by gravity (downhill)
SOIL FORMATION
Soil formation is variable across locations around the world
Heterogeneity
Soil formation is impacted by environmental factors
Highly dependent upon
Geological parent material
Four processes which transform parent material to soil
Soil forming factors
FORMATION OF SOIL
Parent Material - Originating sources of mineral matter
Climate - Temperature and precipitation
Biota - Plant and animal life of region
Topography - Slopes, plains, depressions —> runoff, erosion, infiltration, excess water
Time - Duration material subjected to weathering and other processes
Magma escapes through the seems due to geological events. It becomes rock, parent material (solidifies)
Rock or parent material gets subjected to the soil forming factors. Such as climate, Biota, and Topography, over time. Will eventually form Topography and land masses.
Disintegration through weathering.
Transportation or erosion because of climate. Such as alluvial, eolian, colluvial, etc.
Deposited and mixes with organic matter and then becomes soil
UNIT 3
*SOIL ARCHITECTURE AND PHYSICAL PROPERTIES*
SOIL PROFILE AND HORIZONS
Profile-
Vertical cross-section of soil
Horizons-
Distinct layers withing profile
O-Organic layer
Most biological activity
A-top soil/root zone
90% plant roots and nutrients exist here
B-Subsurface
Fewer roots
Mostly soil with some rock
C-Mostly rock
Almost no organic matter
SOIL COLOR
What influences the color of soil?
Organic matter
Dark/black soils: typically rich in organic matter
Moisture
Moist: darker soil
Dry: lighter soil
Presence of salts and metals
Light soils
Typically calcareous:
High in calcium (Ca)
Can be affected by other salts:
Sodium (Na) and potassium (K)
Oxidation or Reduction of salts and metals
Red soils-
Iron oxide/rust:
Iron (Fe) gaining/reacting with oxygen (O)
Gray/Green Soils-
Iron oxide reduction:
Iron losing oxygen
Iron oxide + carbon —> iron + carbon dioxide
2Fe2O3 + 3C —> 4Fe +3CO2
SOIL TEXTURE
Soil texture-
Determined by the amount of sand, silt, and clay particles in soil
Describes the relative size of soil particles
Particle Sizes (micrometers):
1 micrometer = 0.001 millimeter
Sand > 0.05mm
Silt 0.05-0.002mm
Clay < 0.002mm
Textural Classes:
12 designations of the soil texture triangle
Percentage of sand, silt, and clay in a soil
Physical Characteristics: Soil Particles
Surface area - area of matter exposed to air
Porosity - total volume of pore space
Smaller particles (clay) have
more surface area
greater porosity
micro pores
Larger particles (sand) have:
less surface area
lower porosity
Macro pores
Practically thinking…
What happens in pore space?
Water holding
air exchange
plant root growth
organism habitation
nutrient ionization
Larger pore spaces (Sand):
Increased
water movement
air exchange
Greater porosity (clay):
Increased holding capacity of
water, air, roots, organisms, nutrients
Soil Particles
Sand
Largest particle
No electrical charge
Most macro pores
Best tilth: ability to cultivate
Highest rate of
Drainage, water infiltration, gas exchange
Facilitates root diameter and length
Clay
Smallest soil particle
Only soil particle with a charge
Negatively charged
Exchange of cations and anions
Best holding capacity of:
Water, nutrients, air, organism habitat
Silt
Size between sand and clay
No electrical charge
Similar shape and minerals as sand
High weathering potential
Increase nutrient availability
Soil Structure
Soil Structure-
Arrangement of soil particles into peds/aggregates
Sand, silt, clay, and organic matter
Categorized by:
Type: shape of structure
Size
Strength: penetration resistance
Impacts of Soil Structure
Soil structure can influence:
Water movement
Aeration (air movement)
Root Growth
Heat transfer
Tilth
Micro-organisms
Poor soil structure (compaction) can inhibit or worsen these factors
Improving Soil Structure
Preventing Compaction
Conservation tillage and reduced tractor usage
Mowing weeds instead of discing
Planting cover crops
Avoid walking on when wet
Adding organic matter
Remediating Compaction
Conservation tillage and reduced tractor usage
Deep ripping
Planting cover crops
Soil amendments to break up soil
Adding organic matter
Measurements: of Soil Structure
■ Particle Density- Mass of soil particle by volume
■ Bulk density-
– Mass per unit volume of soil sample
– Measures pore space (compaction levels)
■ Aggregate stability-
– Ability of soil peds/aggregates to retain structure
– Determines soil ability to resist compaction
– Impacted by organic matter content
■ Porosity- Volume of pore space (ideally 50%)
Soil aggregate refers to a cluster of soil particles that bind together to form a stable, larger structure. These aggregates can vary in size and shape and play a crucial role in soil health, affecting water retention, aeration, and nutrient availability. They are formed through biological activity, organic matter, and physical processes.
*UNIT 4 SOIL ORGANIC MATTER*
The Global Carbon Cycle and Soil
Soil’s role in the carbon cycle
Repository for carbon -
Point of accumulation
Source of carbon -
Plant and Animal residues
Sink for carbon -
Carbon from atmosphere
Mitigates greenhouse effect
Carbon sequestration (Collection & Storage/ When C is stored in soil it is inhibited or delayed from entering atmosphere )
Oxidation of organic matter
Role of Soil Organic Matter
OM’s role in soil quality and health:
Formation and stabilization of soil aggregates
Increase soil macroporosity
The ‘glue’ holding particles together
Habitat and food for soil organisms
Cation Exchange Capacity CEC-
Nutrient pools
Slow release of ionized nutrients
WHC-water holding capacity
Buffers soil pH
Organic Matter in the Soil
O horizon: 20-30% OM by weight
Arable soils (AKA Farmable soils)
contain 1-4% OM
Ideal target 5%
15-33% of plant residues become stabilized soil humus
Decomposition of Plant Residues
Green plant residues
60-90% water
Dry plant matter
90% carbon, hydrogen, oxygen
10% mineral nutrients
Cellulose, lignin, hemicellulose, fat (slow to decompose)
Protein, sugar, starch (easily decomposed)
In aerobics soils —>
Organic compounds become
CO2, water, energy, humus
During the oxidation process
Nutrient elements
released into soil
assimilated by plants
In anaerobic soils (without oxygen) —>
Organic compounds become:
methane- CH4
alcohols like ethanol- CH3CH2OH
other organic compounds
Organic matter decomposition slows
Factors Influencing OM Decomposition
Geographical location
Residue particle size
pH
Microbial diversity
Microbial viability
C:N ratio
Temperature
Moisture
The Carbon to Nitrogen Ratio
Influences:
Rates of OM decomposition
Rates of N mineralization and immobilization
Ideal ratio:
25:1
C:N
* Too much carbon slows decomposition and ties up nitrogen
Composting
Practice of creating humus-like materials from raw materials
Requires
Nitrogen (wet or green waste)
Carbon (dry or brown waste)
Moisture
Air
Surface area-outside of soil
Humus
Characteristics:
Dark colored
Heterogenous
Amorphous
Colloidal mixture
Microscopic particle suspended in solution
Composition:
Lignin - wood
Humic Acids
Complex with clay
Function of Humus
High water holding capacity
Enhances soil fertility
Not a true nutrient
Stimulates mineral weathering
Complexing with metallic ions
Increases CEC
Management of OM
Methods to encourage OM accumulation:
Continual addition of organic materials
Cover crops
Animal manure
Crop residues
Retention of crop roots
Maintain adequate Nitrogen
Legumes
Nitrogen fertilizers
Encourage microbial populations
Minimum tillage and/or soil pertubation
Perennial vegetation
UNIT 5 - SOIL WATER
Features of Water
Chemically Considered A Universal Solvent
Solvents: Solution with ability to dissolve other substances -solute
Asymmetric molecular configuration
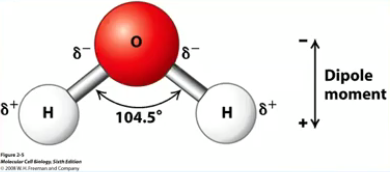
Polarity: Charges are unevenly distributed
H: Electropositive O: Electronegative
Responsible for attraction of other water molecules
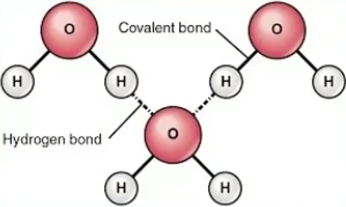
Exists as molecule and compound
Chemical bonding
Molecule
Strong covalent bond
Sharing unpaired electrons
Compound:
Weak hydrogen bond
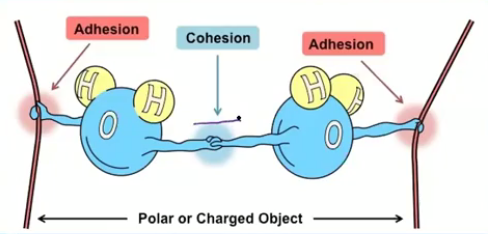
Exhibits both cohesion and adhesion forces
Cohesion-strongly bonds to itself
Adhesion- Bonds to other materials
Enables water to move through soil in any direction
Capillary Movement
Capillary Movement-
Ability of water to move through soil in any direction
Against the force of gravity and plant roots
Occurs in micropores - capillary tubes of soil
Features of Water Continued
Surface Tension-
Tendency of water to behave as if it has a membrane
Result of the high attraction between water molecules (cohesion)
Influences water behavior in soils via capillary movement
Soil Water
Infiltration- Process by which water enters soil profile through precipitation and irrigation
Percolation or drainage- Process by which water moves through soil profile via gravity
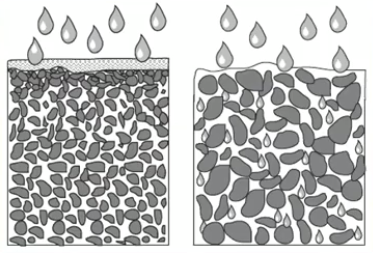
Soil Water Content
Saturation Point- (States of soil water)
All soil pores are full of water
Field Capacity-
Percolation has occurred
Air replaces water in macro-pores
Soil holds optimal water available to plants
Permanent Wilting Point- (PWP)
Water in capillaries of soil
Water unavailable to plants
Hygroscopic Water-
Occurs after pwp
Water molecules tightly held by soil particles (adhesion)
Movement only through vapor
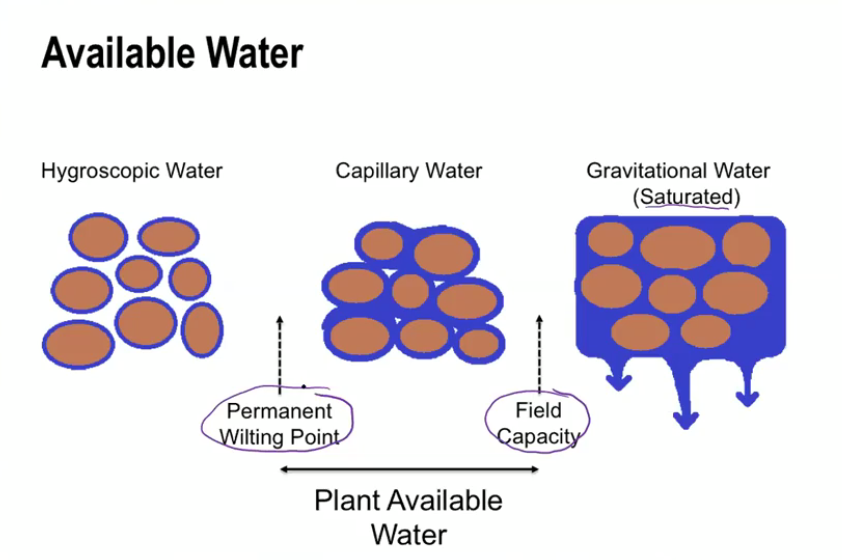
Influences of plant available water
Water holding capacity (WHC)-
Soil’s ability to hold plant - Available water (PAW)
Varies by soil texture (relates to pore space)
Organic Matter Content
Micropores hold water
Higher OM = Higher WHC
Soil Structure - influenced OM content
Impacts infiltration and percolation rates
Relates to OM content
Compaction
Decreases total pore space
Run off
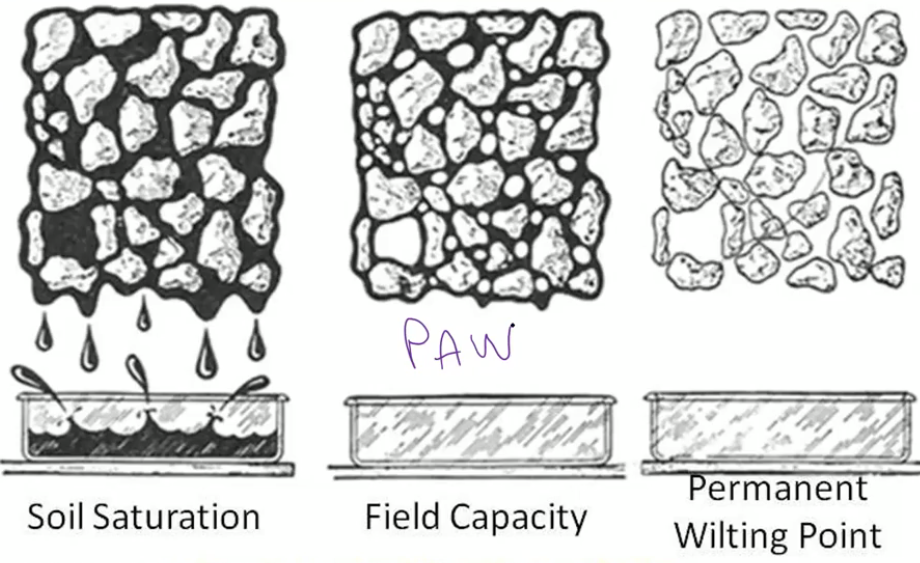
Osmotic Potential-
Soil solute (salt) concentrations
Impacts water movement
Water moves on a gradient
Soil Texture and Water Holding Capacity
(water holding capacity) WHC:
Increases as particle sizes decrease (micropores)
Decrease as particle sizes increase (macropores)
What does this tell you about how texture influences WHC?
In Unit 1 we learned that the ideal water content of soil is 25%. How did the moisture content of the soil tested compare?
The moisture content of the soil tested had a lower percentage that the ideal water content of soil.
If there is a substantial difference, why do you think that is?
Think in terms of seasonal climate and soil properties such as texture and organic matter content.
I think it might be because even though we are in the fall season, there has been extreme heat which may have caused for more drayage of the soil.
What implications does this water content have on soil productivity and its ability to sustain life?
The soil is not very suitable to sustain life since it is too little too dry.
Can you think of any factors that may limit the results of this test? Are there other factors to consider, like season, time of sampling, or any thing else?
As previously mentioned, even if the season is fall, there has been extreme heat which may have factored in.
Do you think this test provides enough information about the soil-water relationship? What is something you learned from lecture or reading that substantiates your position on this?
I do believe that the test provides enough information about the soil-water relationship. I learned from the lecture about the importance of proper moisture for plant growth as well as about porosity, macropores, micropores, and the difference between infiltration and drainage. Meaning that it may be that my soil has an increase in particle size which decreases the water holding capacity of my soil.
Soil Moisture % of a Given Sample
Data Points | Measurements and Calculations |
[A] Crucible weight (g) | 0.043 |
[B] Crucible + wet soil weight (g) | 146.7 |
[C] Crucible + dry soil weight (g) | 124.8 |
[D] Wet soil weight: (B−A) | 146.657 |
[E] Dry soil weight: (C−A) | 124.757 |
[F] Moisture weight: (D−E) | 21.9 |
[G] Soil Moisture: %=100×(F÷E) | 17.55% |
UNIT 6
- Drought reduces snowpack and results in decreased reservoir volume. Here, the effect of drought on 56 of California's more than 700 reservoirs is shown through time.
- Snowpack is an accumulation of snow that compresses with time and melts seasonally, often at high elevation or high latitude. Snowpacks are an important water resource that feed streams and rivers as they melt, sometimes leading to flooding.
How long has California been consistently experiencing drought conditions?
California started being consistently in drought conditions in 2012 but signs were more severe by mid-2013 up until 2017. Totaling up to around 3-4 years.
Based on the data you've observed, what effect has the drought had on our state's reservoirs, snow-pack, and stream flow?
The reservoir volume, as a percentage of total capacity, kept substantially decreasing. The water typically is from the snowpack, meaning that there most likely was also a decrease in wintertime snowpack. Snowpacks feed streams meaning less stream flow.
Based on what you now know about the water cycle, how do you think these conditions have influenced or changed California's water cycle?
What are the primary usages/withdrawals of water in California? What is your opinion on these usages?
Unit 6 - SOil and the Hydrologic Cycle
The Earths Water
The Earths Water exists in:
Oceans
Glaciers
Soil
Deep groundwater aquifers
Exchange between atmosphere and lithosphere
The Water Cycle - Water Returns to the Atmosphere
Evaporation- Vaporation of soil water; returns to atmosphere as gas
Transpiration - Vaporization of water from leaf surface; returns to atmosphere as gas
Evapotranspiration- Total evaporative loss from soil and leaf surface; returns to atmosphere as gas
Condensation- Water droplets bonding together to form clouds
Precipitation- Water released from clouds as rain, sleet, or snow
Infiltration- Water entering the soil; responsible for groundwater recharge
Runoff- Water flowing over the landscape; creates watersheds
Hydrologic Cycle- Cycling of water from earths surface to atmosphere driven by solar energy
Watershed - An area of land drained by a system of streams
ex. San Joaquin Delta Watershed
Water Table- The depth at which groundwater can be accessed
SOIL AND THE WATER CYCLE
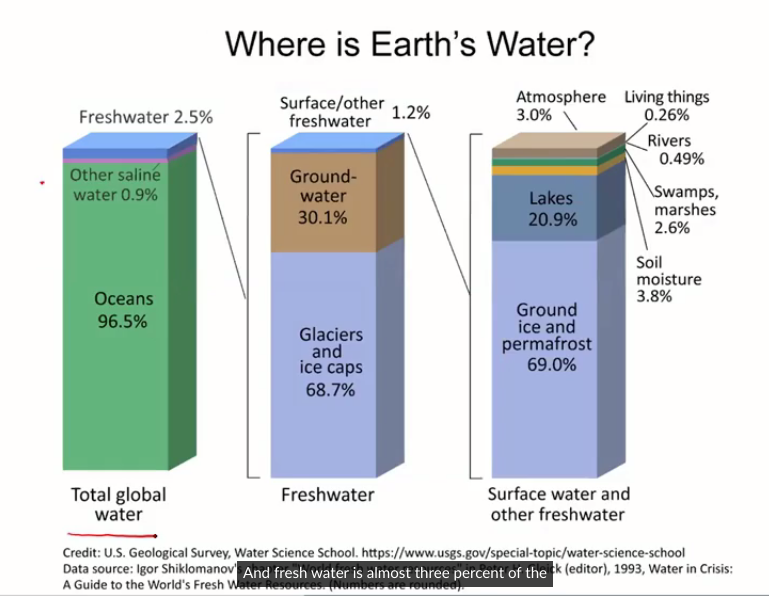
Groundwater
Portion of water cycle resides in soil via infiltration
Soil pore space
Point of access for plants
Water bearing rock
Point of access for humans- wells
Soil properties and management impact how water behaves.
Gravitational water percolates through soil profile
Can carry solutes with it
Nitrogen and Phosphorus
Fertilizers and Ag byproducts
Heavy metals and pollution
Sodium
Water returns to the atmosphere from the soil via:
Evaporation
Transpiration
Roots take IP soil-water
Releases through openings on leaves called stomata
Evapotranspiration
Soil properties and management impact how water behaves
Runoff
Removes topsoil (erosion)
Deposits runoff into bodies of water
Can contain solutes and pollution
Unit 6 Lecture 1
its difficult to measure with varying depths and areas, its always in flux between the atmosphere and lithosphere, its always in varying phases of matter
30.1%
True
False
Lecture 2
Evaporation of water from soil surface, Evaporation of water from leaf surfaces due to transpiration, Quantifiable measurement of total evaporative water loss
water entering the soil vs running over it
True
Lecture 3
How does soil structure and texture influence infiltration, percolation and runoff?
Infiltration is affected by the soil structure and texture since depending on the texture there is a higher level of infiltration such as in sandy due to its loose structures, and lower with clay. Consequently, clay has the most runoff. In silt soils there is higer percolation than in clay but slower than sandy. Silt typically is moderate and in between of sandy and clay texture.
UNIT 7 — SOIL AERATION AND TEMPERATURE
Soil Composition
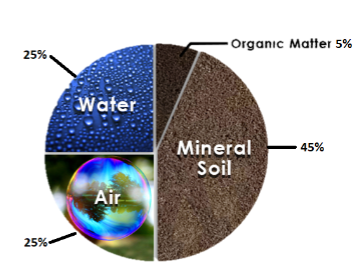
Mineral Matter
Organic Matter
Water
Air
What is Air?
Gaseous envelope surrounding the earth
Commonly referred to as air
The gas called “air” is:
A mixture of gasses
Similar to that found in soil, but
Air Composition
Earth-
78% nitrogen (N2)
21% oxygen (O2)
0.04% Carbon Dioxide (CO2)
Soil-
78% Nitrogen (N2)
20% oxygen (O2)
CO2 can be 10x greater than atmosphere
Air Composition
Atmospheric and Soil Air Contains:
Trace amounts of other gases- <1%
argon, freon, neon, helium, methane
Water vapor- Remember the ater cycle?
Air Composition
Air pollutants
Smog- combusted fuels reacting with solar radiation
Soot- Particulate matter
ash
dust
pollen
mold
Soil Aeration
Aerated soils- constant gas exchange with atmosphere

Air movement:
Mass flow- movement of gas related to pressure and temperature
Diffusion- gradual dispersal of gas molecules to lower concentrations
Why is soil air important?
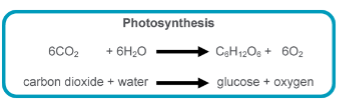
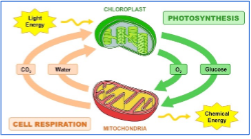
Gas exchange with atmosphere necessary in biological processes:
Photosynthesis
Cellular respiration
The ratio of CO2 and O2 in soil determines its suitability for life
O2 crucial in ionization and mineralization of elements
Ionization- Atom that has gained an electrical charge either through loss or gain of electrons
Mineralization- The release of minerals-plantessential nutrients through the decomposition of OM
Soil organisms:
Require O2- for respiration
Will use other elements in its absence
Ex. iron, nitrogen, sulfur, carbon
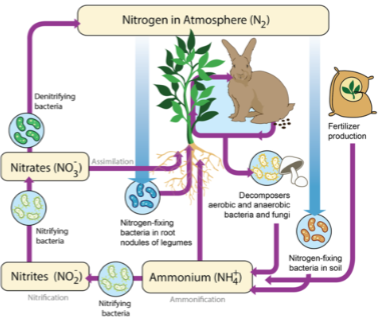
Responsible for ionization and mineralization of plant nutrients via respiration
Ex. nitrogen cycle
O2 influences decomposition of OM
Aerobic vs Anaerobic
With or without O2
Soul organisms present
Mineralization
Aeration allows water to move through profile
Infiltration, percolation, run-off
Water carries nutrients
Aeration allows plant root development
Pore space
O2 uptake via root
Gas concentrations determine form and availability of plant essential nutrients.
Presence of O2 impacts redox reactions
Redox-
Reduction- gain electrong
Oxidation- loss of electrong
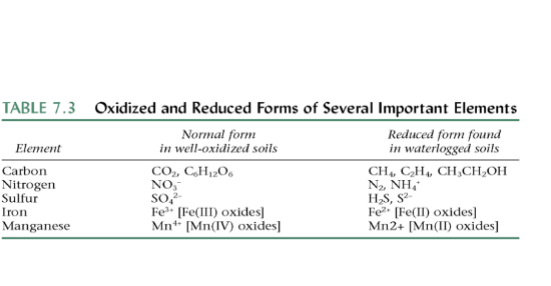
^Fe and Mn can be toxic in reduced form, or unavailable in oxidized form
Factors Affecting Soil Aeration
Soil heterogeneity-
Macropore volume
Soil texture, structure, density
OM content
Presence of deep cracks
Higher O2 concentrations in upper horizons
Drainage-
Air cannot occupy pores filled with water
Hardpan/compaction
Water table depth
Porosity
Organism respiration rates-
Use O2 and CO2
Impact diffusion rates
Plant roots-
Decrease soil O2
Remove soil water
Soil management-
Tillage
Increase aeration short-term
Can lead to compaction
lower aeration over time
OM sequestration
Management to improve soil aeration
Improve drainage and avoid compaction-
Add and retain OM
Avoid working wet soil
Conservation tillage and equipment use
Deep ripping
Aeration equipment
Measuring Soil Aeration Status
Redox: oxidation-reduction potential of soil
Volume of macro pores
CO2 and O2 levels
Soil Saturation- pores occupied by water cannot be occupied by air
Soil Temperature
Temperature impacts biological, chemical, and physical properties of soil
Biological-
Plant processes
Seed germination
Root function
Shoot growth
Warm vs cool season
Biological and Chemical-
Microbial Processes in warm temps
Increase respiration
Alter soil aeration levels
Impacts nutrient cycling
Microbial Processes in cool temps:
Lower decomposition rates/mineralization
Lower ion oxidation
Impacts nutrient cycling
Physical-
OM Content
Influences soil structure
Freezing and thawing
Increases granular structure
Factors Influencing Soil Temperature
Solar radiation
Primary source of soil heat
Specific heat of soil
Dry soil requires less energy to heat
Vaporization of soil water
Evaporation cools soil
Fire
Temporary heating of soil
Managing Soil Temperature
Insulate soil with mulch
Living, organic, plastic
Solarization
Provide adequate drainage and aeration
Raise soil level
“Bed-up” soil: Manteca farm example
Raised beds
Unit 7 Lecture 1
Gaseous mixture known as air. Gaseous envelope surrounding earth.
CO2
it contains water vapor
driven by temperature vs. gas concentrations. movement in large volume vs. gradual dispersal
Unite 7 Lecture 2
Photosynthesis. Cellular respiration.
increase of plant available nutrients through cellular respiration
slow or cease ionization, slow decomposition of OM and mineralization, decrease cellular respiration, substitute other nutrients for O2
False
Unit 7 Lecture 3
False
Pore space
Oxidations is losing electrons, Determine form and availability of plant nutrients, Reduction is gaining electrons, Related to the sharing of electrons
UNIT 8 - SOIL COLLOIDS
Colloidal Particles
Colloid-
Microscopic particles dispersed in solution
Particles will not settle
Organic and inorganic
Soil Solution- Aqueous / water phase of soil contains dissolved organic & inorganic particles (colloids)
Soil Colloidal Particles
Soil colloids-
Organic and inorganic particles in soil solution
Very small —> <2mm
High surface area
Highly reactive
Types of Soil Colloids
Inorganic Colloid- the clay fraction of colloids MM
Crystalline Silicate Clays
Non-crystalline Silicate Clays
Iron and Aluminum Oxides
Organic Colloid- the humus (carbon) fraction OM
Soil Colloid Properties
High surface area- Concentration of micro pores:
Electrically charged surfaces
Increase water holding capacity
Microbiological activity
Nutrient formation and ionization
Aeration and gas exchange
Root growth
Highly reactive- Carry negative and positive charges
Negatively charged surfaces
Adsorb- Attraction of ions, including H2O, to colloid surfaces
ability to attract and hold nutrient ions
Cation Exchange Capacity (CEC)
Cation exchange- cations moving between colloidal surfaces and soil solution
CEC- Measurement of the total quantity of cations able to adsorb to soil particle surface -Nutrient holding capacity
In other words…CEC measures how fertile or potentially fertile a soil is, or quantifies the Nutrient Pool!
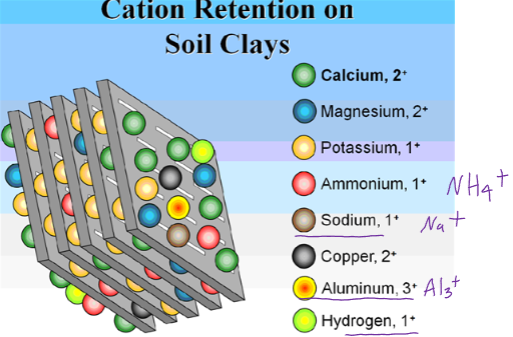
Two major problems associated with low CEC
Nutrients not adsorbed can become pollutants
Nitrate (NO3)
Phosphate (P2O5)
Nutrients —> fertilizers:
Fertilizers are costly and can be overapplied
Colloids and CEC
CEC expressed in values 0-250 cmol/kg (molecular/mass)
Organic Matter (OM) -humus
150 to 250 cmol/kg
Clay
10-50 cmol/kg
Practically thinking, what would you do to improve soil CEC??
Nutrient Pools
Measurable state of plant nutrients in the soil
—> quantifiable
Soluble- Readily available = dissolved into solution
Exchangeable cations (CEC)
Carbonates, sulfates, chlorides ionized nutrients
Insoluble- Available over long periods of time —> slow release fertilizer
OM- mineralization yet to occur *raw materials*
Feldspars, apatite, mica —> primary minerals * raw materials*
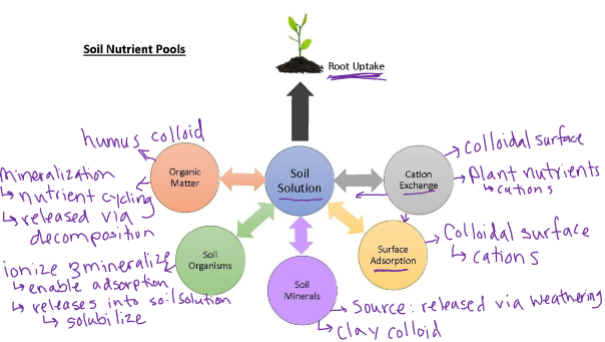
Soil Nutrient Relationships
Possible fate of soil nutrients:
Plant root uptake
Cycling through plant development and decomposition
Removal through harvest and tillage
Holding in soil colloids - adsorption
Leaching and runoff - Nutrients are carried by water either through percolation or via runoff
Unit 8 Part 1
True
Organic
High surface area
False
Unit 8 Part 2
what would you do to improve soil CEC??
In order to improve my soil CEC I would add organic matter to it such as humus. Which will consequently create fertility in the plant, prevent pollutants, and be a cheaper method than utilizing fertilizer.
Unit 8 part 3
soluble
OM, Mineral Matter, CEC, Surface adsorption, Soil Organisms
UNIT 9 SOIL ACIDITY, ALKALINITY, AND SALINITY
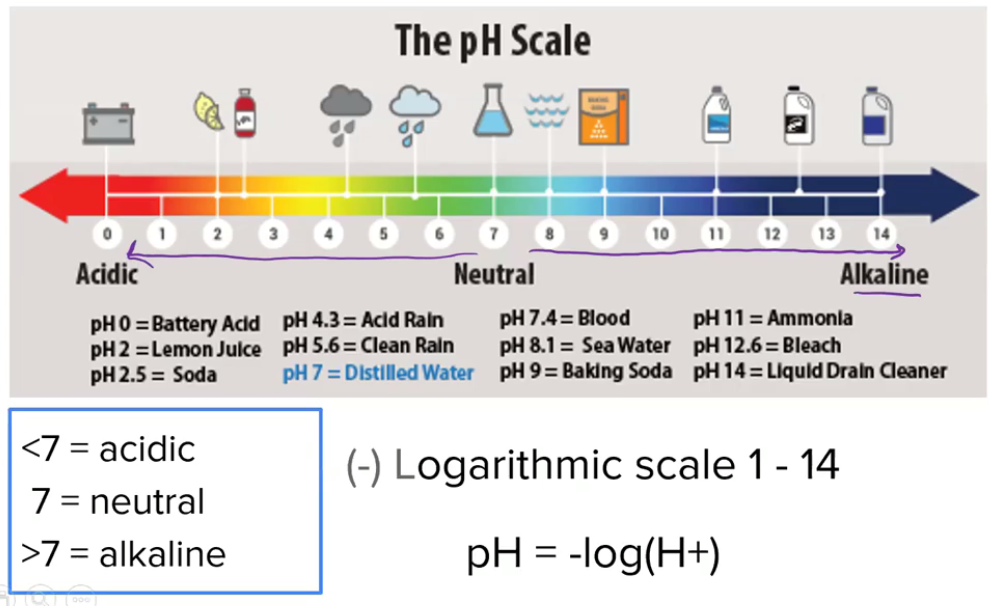
pH
Measurement of hydrogen ion (H+) concentration
H+ increases/decreases by 10x with each level
Ex. increase of pH from 5 to 7 increases H+ exponentially by 10²
Indication of hydroxyl ion (OH-) ion concentration
Inverse relationship with H+
Soil pH
Degree of acidity or alkalinity in a soil
Acid VS base forming ions
Master variable in chemical reactivity
Affects all soil properties
Chemical
Biological
Physical
Agricultural soils ideal range: 6.2-7.3
The importance of pH
Determines: Chemical Properties of Soil
Ion availability and CEC
pH influences whether nutrient levels are:
Optimum, Deficient, or Toxic
Increase in pH increases CEC
OH- on colloid surfaces exchange H+ and other cations
Mobility of soil pollution
rate of biochemical breakdown
solubility
Adsorption
Toxins destroyed in the soil VS Toxins passed into groundwater
Determines: Biological Properties of Soil
Soil suitability for life
Which plants dominate the landscape
Root uptake availability
most prefer neutral
Activity of microbes
Presence of fungi
Determines: Physical Properties of Soil
Soil structure
Alkalinity causes particles dispersal
Associated with Na+ (sodium)
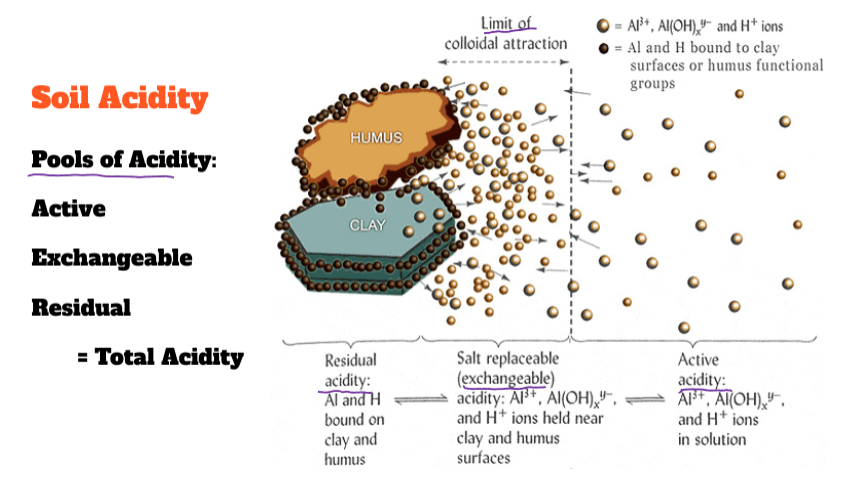
Soil Acidity
Result of:
Production of H+ ions
Rain water
Accumulation of OM
Oxidation of N and S
Plant uptake of …
Leaching of base ions via percolation
High precipitation
(Percolation + soil solids = leaching)
The role of Aluminum (A13+)
Adsorbed-
H+ releases A13+ from minerals
(colloid via wethering)
A13+ cleaves water molecule
Frees H+ to acidify soil
Causes toxicity in plants
Soil Alkalinity
Result of reactions that consume(bonding w/anion) H+ or produce OH-
Weathering and accumulation of base forming cations
calcium (Ca2+), sodium (Na+)
Weathering consumes H+
Low precipitation
Avoid leaching
Production of base forming anions
carbonates (CO3)
bicarbonates (HCO3-)
React with water to form OH-
pH and Soil
pH problems are chemical imbalances-
Excessive fertilizers
Loss of OM
Poor farming practices
Biological activity
Acid rain
Pollutants
Buffering
Soils ability to resist pH change when acid/base is added
Mechanisms-
Cation exchange react
A13+ reactions
OM reactions
H+ on clay colloids
Carbonate reactions
Importance of Soil Buffering
Maintain stable pH environment
Soil biota
Influences agricultural management
process of neutralizing acid or base environments or correcting a chemical imbalance
Buffer- Material that will neutralize an acid or base
Amendment- Addition to the soil which is incorporated into the profile
Correcting Acidic Soils
Amending to buffer acidic soils-
Add Ag Lime (limestone) - calcium carbonate (CaCO3)
Add Dolomite Lime- calcium carbonate and magnesium carbonate (CaCO3MgCO3)
Correcting Acidic Soils: How it works…
CaCO3 (+) clay
H+Clay
Ca2+ (+) HCO3
Ca+ and/or Mg+ cations replace H+ cations on soil colloids
Carbonate (CO3) reacts with H+ to form bicarbonates (HCO3-)
The bicarbonate dissociates in water forming OH-ions
The H+ is consumed raising pH
Correcting Alkaline Soils
Add OM
Add sulfur- two chemical reactions associated with sulfur buffering
Involves water: 2S + 302 + 2H2O —> 4H + 2SO4
Oxidation reaction:
microorganisms
metabolize sulfur and release sulfate
sulfate reacts with water to produce sulfuric acid = lowered pH:
Salinity and Sodicity
Defined: concentration of salts dissolved in solution
What are salts?
Chemical definition: solid crystalline structured precipitate formed through an acid/base reaction
Thousands of salt types
vary in color, composition, reactivity, solubility, and other physical and chemical …
Salts are formed by cations and anions-
They are potential plant nutrients
Can be toxic in excess concentrations
Plant nutrient salts
Ca, K, Mg
Toxic/detrimental salts
Al, Se, Cl, Na
Saline(desert) Soils- Ca, K, Mg, Al, Se, Cl
Sodic Soils(salt water encroachment)- Na (sodium)
Management of Saline Soils
Plant salt tolerant crops
Sugar beets, wheat, barley
Improve DRainage- Leaching
Amend soil with gypsum CaSO4
SO4 bonds with Na
Leaches through profile
Deep ripping
Raise beds
Apply pure water to leach slats
Salts are measured using electro-conductivity (EC)
Salts conduct electricity; can easily be measured in solution
U.9 L.1
As pH values decrease = H+ ions increase, Solution becomes more acidic
pH is a measurement of H+ and indicator of OH- due to their ___ relationship = Inverse
Due to its influence on the chemical properties of solutions, pH is termed a Royal Variable = False
U.9 L.2
CEC increases as pH = increases
What is the ideal pH range for agricultural soils? = 6.3-7.3
What are the primary effects of pH on soil chemical properties? = ionization, CEC, pollution mobility
pH determines a soils suitability for life = True
U.9 L.3
Select the following ways that H+ ions are produced in the soil = rain water mixing with CO2, OM accumulation, Oxidation of N and S, Plant uptake
How does precipitation contribute to acidification = percolation + soil slids = leaching
A13+ contributes to soil pH because it is an acid forming ion = false
U.9 L.4
What does ‘consuming’ H+ mean? = H+ bonding with an anion, H+ becomes unable to further acidify a solution
Bicarbonates alter pH because they react with water to form OH- = True
What is the difference between calcareous and sodic soils? = high in calcium salts vs. sodium salts
U.9 L.5
How does fertilization alter soil pH? = all answers are correct
Cation exchange and colloids play a role in soil buffering capacity because accumulation of acid and base ions is responsible for pH changes = True
Compare and contrast buffers and an amendments? = buffers neutralize acids and bases, amendments can be buffers, amendments are added to the soil profile
Calcium and magnesium are plant nutrients and are directly responsible for changing pH = False
What are the differences between sulfur buffering reactions in the soil? = involving water vs. oxidation, oxidation commonly occurs naturally, water reaction with S is a common agricultural practice
UNIT 10 Organisms and Ecology of the Soil
The Soil Ecosystem
Ecosystem- Complex systems with biodiversity
Key terms-
Biodiversity- the wide variety of organisms in a system
Fauna- general term for animals
Flora- general term for plants and non-animal organisms
The Soil Ecosystem and its Organisms
Flora and Fauna-
Macro > 2mm
Meso 0.1-2.mm
Micro < 0.1mm
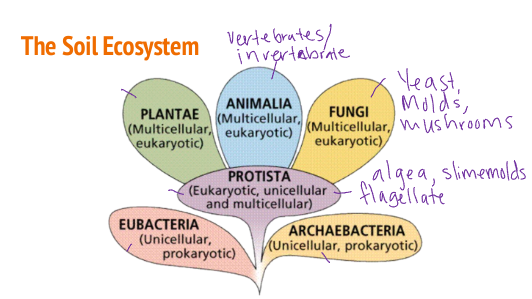
Eukarya-
Organisms with cellular nucleus and organelles
Multicellular and unicellular
Plants, animals, fungi, protists
Prokarya-
Organisms without cellular nucleus and organelles
Unicellular
Bacteria
THE SOIL FOOD WEB
Food Web-
Complex system of interdependent food chains
Many organisms are omnivorous
Organisms grouped by their sources of carbon and energy
Trophic Levels- Consumption, energy/carbon sources
Primary Producers
Primary Consumers
Decomposers
Secondary Consumers
tertiary consumers
First trophic level-Primary Producers
Autotrophs:
Photoautotrophs:
Rely on CO2 for carbon
Photosynthesis for energy
Plants, phytoplankton
Chemoautotrophs:
Rely on CO2- or CO3- for carbon
Oxidation of NH4+, S, Fe for energy
Some bacteria
Second trophic level- Primary Consumers
Heterotrophs:
Rely on organic compounds for energy and carbon
Herbivores:
Consume plants
Nematodes
Insects-termites, larva
Shredders- rodents, earthworms
Detrivores: decomposers
Consume decaying plant matter
Insects, fungi, bacteria
Fungivores: Consume fungi
Bacterivores: Consume bacteria
Saprotrophic: Consume dead tissue
Third trophic level-Secondary Consumers
Low level Predators and Parasites:
Consume primary consumers
Omnivores and carnivores
Carnivores- Centipedes, mites, bacteria, fungi, nematodes, spiders, snails
Parasites- mites, bacteria, fungi, nematodes, protozoa don’t consume prey; feed off of host
Fourth and Fifth trophic level-Tertiary Consumers
Higher Level Predators:
Consume secondary consumers
Omnivores, Obligatory Carnivores
Ants, spiders, scorpions, birds, moles
Functions of Soil Organisms
Nutrient Cycling
Formation of Soil Structure
Weed Suppression
Inhibiting Pests and Diseases
Carbon Sequestration
Healthy soil organisms —> Nutrient cycling:
Improve and support healthy plant growth
Provides nutritional value of crops
Sustains agricultural and natural systems
Supports and maintains biodiversity
Provides ecosystem stability
Nutrient Cycle
Sequestration-
collection of nutrients to soil
Cycling-
Mineralization
Bioturbation
Transformation-
Ionization
Assimilation-
Solubilize nutrients for plant uptake
Healthy soil organisms —> Suppress Weeds:
Functioning nutrient cycles —> nutrition for crops
NO3- feeds weeds
NH4+ feeds crops
Nitrifying bacteria convert:
NH4+—> NO2—>NO3
Fungi predates on bacteria
Healthy soil organisms —> Inhibit pests and diseases:
Functioning nutrient cycles —> healthy plants
Healthy vigorous plants resist insect pests and pathogens
Sustained biodiversity —> microbial populations
Microbes
Suppress soil borne pathogens
Predate on pathogenic species
Actinomycetes
Formed Soil Structure —> improved aeration
Aerobes-
Organisms require high volumes of O2
Mostly beneficial
Anaerobes-
Organisms do not require high volume of O2
Mostly detrimental
healthy soil organisms —> Sequester carbon:
Soil Macrofauna
Role of macrofauna-
To mix organic matter with soil
Complex MM with OM
To increase aeration through channeling and burrowing
To accelerate decomposition of organic matter
Role of Macrofauna
Macrofauna and the acceleration of OM decomposition
Springtails, termites, fly larvae: Puncture leaf epidermis and open leaf to microorganisms
Mollusks, termites, millipedes, earwigs, fly larvae, earthworms eat OM, pulverize it through excretion
Earthworms, insects, and burrowers transport the OM into soil
Earthworms and potworms consume OM in soil and further mix it
Microbes take over from there
Types of Macrofauna
Earthworms-
Consume detritus
Do not harm plants
Increase macroporosity
Drainage, infiltration, aeration, root penetration
Increase availability of nutrients and aggregates
Castings- partially digested OM and soil
Common Types of Macrofauna
Termites- feed on carbon
Springtails- detritivores
Mollusks- feed on plant material
Earwigs- feed on plant material
Pill Bugs- detritivores
Millipedes- Omnivores: detritus, fungi, plant juices, some predatory
Soil Fungi
Mycology- Study of fungi
Myco- Prefix for fungi: Greek root word ‘Mykes’
Includes:
Mold, mildew, rusts, smuts, yeast, puffball, mushroom
Multi celled- Eukaryotic
Mostly consume detritus
Micro and macro floral species
Role of Fungi in Soil
Functions of fungi (bacterial)
Antibiotic production
Decomposition of OM
Predate on insects, fungi, bacteria
Nutrient cycling and formation
food source
degradation of pollution
Mycelium- Roots of fungi
Mycorrhizae— A group of beneficial fungi living symbiotically on plant roots
Increase root surface area- extended access to water and nutrients
Element ionization —> nutrient formation
Provides tolerance for plant against salts, pesticides, diseases, parasites
Some deliver nutrients to plants
Soil Microfauna
Role of microfauna-
Decompose OM
Mineralization
Nutrient ionization
Pollution degradation
Control populations
Predation and parasitization
Nematodes-
Microscopic unsegmented worms
Found in almost all soils
Can be beneficial or agricultural pests
Impacts fungal and bacterial populations
Alters nutrient cycling
Food sources:
Fungi, bacteria, algae, plants, nematodes, protozoa, insect larvae
Soil Organisms- Bacteria
Bacteria-
Single celled
Prokaryotic
Measured in:
Micrometers
Gram positive vs gram negative
Morphology
Cocci- spherical shape
Bacilli- rob shaped
Spirilla- tightly coiled or spiraled
Colonies
Staphs- colonize in clusters
Filamentous- form filaments
Form biofilm networks
Bacteria
Found in most habitats on earth
Largest populations and species diversity exist in the SOIL
Healthy soil —> 40-50 million bacteria per gram
<10% of bacteria is pathogenic (disease causing)
Roughly 90% of bacteria is beneficial or benign (harmless)
Bacterial Ecological Fuctions-
Recycling nutrients
Key role in Nitrogen fixation and cycling
Decompose animal proteins
Predation/parasitism manages populations
Antibiotic production help plants manage diseases
Actinomycetes key antibiotic producers
U.10 L.1
The soil ecosystem is diverse because all kingdoms of life are represented there = True
What is the characteristic which makes eukaryotic cells unique? = possess organelles
Select the characteristics which may prokaryotic cells unique = do not possess organelles, unicellular
U.10 L.2
A food chain is a complex system of interdependent organisms = false
What is the primary characteristic of autotrophs? = they synthesize their own carbon
What is the primary characteristic of heterotrophs? = they rely on other organisms for their carbon
What is the primary characteristic of detritivores? = they consume decomposing carbon
Predators and parasites both keep populations of other organisms regulated by feeding and infecting them. = True