Atomic and Nuclear Physics-Chapter 7(HL)
7.1 Discrete Energy and Radioactivity
Discrete energy:
Emission spectrum represents different possible wavelengths of light emitted by an atom.
When gas at low pressure is subjected to a strong electric field, it emits light at discrete wavelengths.
The emission spectrum (e.g., hydrogen, helium, mercury) comprises lines at specific wavelengths, representing photon emissions during electron transitions between energy levels.
Emission spectrum: Series of bright lines representing the wavelengths that can be emitted by an atom.
From the excited state, the electron will immediately (within nanoseconds) make a transition down to one of the available lower energy states.This process is called relaxation.
, h is planck's constant, is the wavelength emitted during relaxation and c is the speed of light, whereas E is the energy released.
This means that the light that is transmitted through the gas will be missing the photons that have been absorbed.This gives rise to absorption spectra
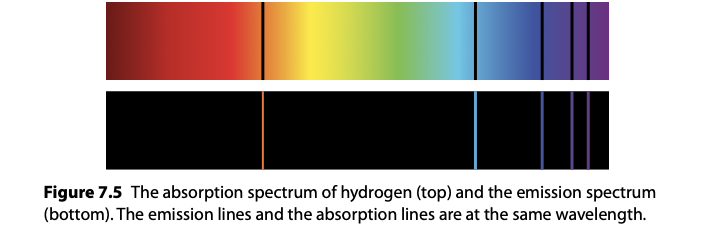
Radioactivity: Spontaneous emission of particles and energy from an unstable nucleus.
Discovered by Henri Becquerel, Marie Skłodowska-Curie (1867–1934), and Pierre Curie (1859–1906).
Alpha particles: Helium nucleus emitted during alpha decay.
Beta particles: Electrons or positrons emitted during beta decay.
Gamma rays: High-frequency electromagnetic radiation from nucleus transitions.
Nuclear transmutation: Transformation of one element to another through nuclear reactions, such as alpha particle collision with nitrogen to produce oxygen and a proton.
Nuclear fission:
Splitting of a heavy nucleus into lighter nuclei, with the release of energy.
Example: Absorption of a neutron by uranium-235, resulting in uranium-236, which then fissions into krypton, barium, and more neutrons.
Chain reaction: Self-sustaining fission process due to released neutrons inducing further reactions.
Critical mass: Minimum mass of fissile material needed to maintain a chain reaction.
Nuclear fusion:
Joining of light nuclei to form a heavier nucleus, releasing energy.
Occurs in stars and hydrogen bombs, but controlled fusion for energy production is still a challenge.
Nuclear structure: Constituents of a nucleus (protons and neutrons) and their organization.
Atomic (proton) number (Z): Number of protons in a nucleus.
Mass (nucleon) number (A): Sum of protons and neutrons in the nucleus.
Neutrons (N): Calculated as N = A - Z.


Isotopes: Atoms with the same number of protons but different numbers of neutrons. Physical properties vary, but chemical properties are identical due to the same number of electrons.
Radioactive isotopes: Exhibit spontaneous radioactive decay.
Radioactive decay:
Unstable nuclei emit particles and energy spontaneously.
Types of decay: alpha (α), beta (β), and gamma (γ) radiation.
Alpha decay: Emission of an alpha particle (helium nucleus).

Beta decay: Neutron transforms into a proton emitting an electron (beta-minus decay) or proton transforms into a neutron emitting a positron (beta-plus decay).
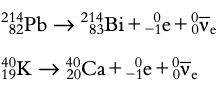

Gamma decay: Emission of a gamma ray, no change in the nucleus's atomic number or mass number.


Half-Life & Probability:
Half-life (t1/2): The time required for half the quantity of a radioactive substance to undergo decay. A measure of the stability of a radioactive isotope; shorter half-life indicates a more unstable isotope. Determines the rate at which a sample loses its radioactivity.
Exponential decay: The number of undecayed nuclei decreases exponentially over time.
Probability in decay:
Each nucleus has a constant probability of decaying in a given time interval, independent of time.
After one half-life, the probability that a nucleus has not decayed is 50%.
Multiple half-lives follow a predictable pattern: after n half-lives, the fraction remaining is
Decay Series
Radioactive decay series: A sequence of decay events from a parent radionuclide to stable daughter isotopes.
Example: The decay series of uranium-238 to lead-206 involves multiple alpha and beta decays.
Each step in the series has its own characteristic half-life and decay mode.
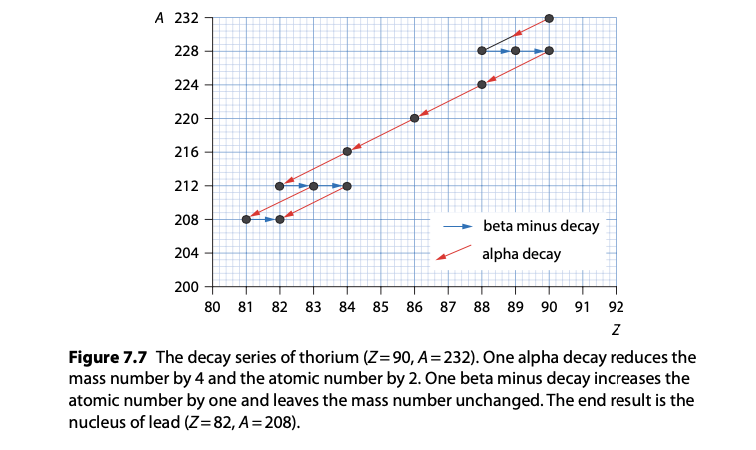
The Law of Radioactive Decay
Radioactive decay law: States that the activity (rate of decay) of a radioactive sample is proportional to the number of undecayed nuclei present at any time.
Mathematically expressed as
, where N is the amount of undecayed nuclei.
7.2 Nuclear Reactions
Transmutation and energy release:
Unified atomic mass unit (u): A standard unit of mass that quantifies mass on an atomic or molecular scale.
1 u is defined as one twelfth the mass of a carbon-12 atom, approximately
1.660539×10-27 kilograms.
Mass Defect and Binding Energy
Mass defect (Δ): The difference between the mass of the completely separated nucleons and the mass of the nucleus.
Occurs because mass is converted into binding energy when the nucleus forms.
Formula:
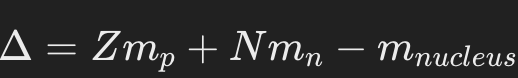
Where is Z is the number of protons, N is the number of neutrons and and is the mass of the proton and mass of the neutron respectively, whereas is the actual mass of the nucleus.
Binding energy: The energy required to disassemble a nucleus into its individual protons and neutrons.
Calculated using Einstein's equation
m is mass defect often given as a lower case delta, and c is the speed of light.
The Binding Energy Curve:
The binding energy per nucleon varies with the nucleon number and has a peak at iron-56, indicating the greatest stability.
Light nuclei (up to iron) gain stability through fusion, while heavy nuclei (beyond iron) gain stability through fission.
Energy Released in Decay
Nuclear fission: A heavy nucleus splits into two smaller nuclei, releasing a large amount of energy.
Example: Uranium-235 undergo fission after capturing a neutron.
Energy released is due to the conversion of mass defect into energy, typically measured in mega-electron volts (MeV).
Nuclear fusion: Lighter nuclei combine to form a heavier nucleus, releasing energy.
Example: Fusion of deuterium and tritium to form helium-4.
Requires high temperatures and pressures to overcome electrostatic repulsion, with the sun being a natural fusion reactor.
7.3 The Structure of Matter
Particle physics:
Investigates fundamental building blocks of matter (quarks and leptons) and their interactions.
Rutherford experiment: Revealed the nucleus and led to the planetary model of the atom.
Fundamental particles:
Quarks: Six types ('flavors') with different properties, combining to form particles like protons and neutrons.
Leptons: Include electrons, neutrinos, and their anti-particles, not subject to the strong interaction.
Exchange particles: Mediate fundamental forces (e.g., photons for electromagnetic force).
Nuclear Forces and Particles:
Strong nuclear force: Binds quarks within protons and neutrons, and these nucleons within the nucleus.
Alpha, beta, and gamma decay: Processes by which unstable nuclei release particles and energy.
The Higgs boson: Particle associated with the Higgs field, which gives mass to other particles in the Standard Model.
Exchange Particles and Fundamental Forces:
Electromagnetic interactions: Mediated by photons.
Weak interactions: Involve W and Z bosons, responsible for processes like beta decay.
Strong interactions: Governed by gluons, binding quarks together within nucleons.
Gravitational interactions: Attributed to gravitons, though not yet experimentally confirmed.
Conservation Laws in Particle Physics
Baryon number: Conserved in nuclear reactions; associated with quarks and baryons.
Lepton number: Conserved for electrons, muons, and their respective neutrinos.
Strangeness: Quantum number conserved in strong interactions, may change in weak interactions.
Electric charge: Conserved in all types of interactions.
Feynman Diagrams:-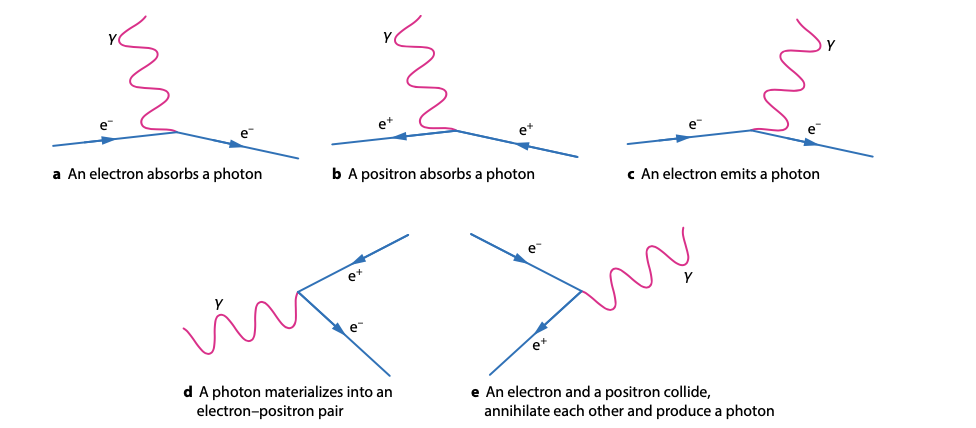
Visual representations: Depict particle interactions, with particles as lines and interactions as vertices.
Interaction vertices: Show the exchange of force carriers like photons and W/Z bosons.
Important for calculations: Simplify understanding of complex interactions in quantum field theory.
Exam Tips
Be prepared to apply knowledge of discrete energy levels and transitions to solve problems.
Remember to convert eV to joules for energy-related calculations.
Understand the significance of the binding energy curve and its implications for nuclear stability.
Be familiar with Feynman diagrams to represent particle interactions and decays.