Week 9: Blood Examination & Properties
Lecture part 1 + 2. finished.
BLOOD PROPERTIES
Volume: 6-8% of body weight
pH: Approximately 7.4
Color:
Arterial blood: Red
Venous blood: Dark red
Odor: Specific
Taste: Slightly salty
Functions of Blood 🩸
Blood serves three primary functions:
Supply:
Oxygen
Essential nutrients
Enzymes
Hormones
Water and electrolytes
Buffering system to maintain constant conditions
Removal: Metabolic waste products
Defense:
Blood cells (leukocytes)
Blood proteins (immunoglobulins - antibodies)
Blood Composition 🧬
Blood consists of two main components:
Plasma: Contains fibrinogen
Blood corpuscles
Blood Cells (Static) Forming Organs 🩸
Hematopoiesis: The process of blood cell formation and development.
All blood cells originate from the stem cell called the hemocytoblast.
Hemocytoblast → Erythroid stem cell → RBC (if becoming a red blood cell)
Forming organs:
Bone marrow
Liver
Spleen
Lymph nodes
Reticulo-endothelial tissue
Functions of the Spleen 🫘
Blood reservoir: Storage site for red blood cells and platelets; the body can contract the spleen to release more red blood cells.
Site of immune activities: Part of the immune system.
Blood filtration: Removes old or abnormal blood cells and particles.
Hematopoiesis: Produces certain blood cells and is a major site for red blood cell production outside the bone marrow.
Phagocytosis: Traps and removes old cells, bacteria, and foreign proteins from circulation.
Haematology 🧪
Study of blood and blood-forming organs.
Haematological Parameters
Red Blood Cell count (RBC): [Tera (10^12)/l]
White Blood Cell count (WBC): [Giga (10^9)/l]
Packed Cell Volume (PCV): [% or l/l]
Haematocrit (HCt): [l/l]
Haemoglobin (Hb): [g/l or g/dl]
Mean Cell Volume (MCV): [fl]
Mean Corpuscular Haemoglobin (MCH): [pg]
Mean Corpuscular Haemoglobin Concentration (MCHC): [mmol/l or g/dl]
Platelets count: [Giga (10^9)/l]
Differential WBC: (lymphocytes, monocytes, neutrophils, eosinophils, basophils) [% and Giga (10^9)/l]
Reticulocytes count: [‰]
Haematological Examination Methods
Routine methods
Electrical impedance methods
Flow cytometry methods
Blood Sampling Conditions 🩸
Avoid excitement
Disinfect the venipuncture site
Use dry needles (to prevent hemolysis)
Limit pressure for vein distension to < 2 minutes (to avoid haemoconcentration, hemolysis)
Blood Sampling Sites by Animal 📍
Cattle: Jugular vein, subcutaneous abdominal vein (anterior mammary), middle coccygeal vein
Small Ruminants: Jugular vein
Horse: Jugular vein
Pigs:
Small pigs: V-shaped trough
Large pigs: Loop noose around the snout
Ophthalmic (orbital sinus)
Puncture of ear vein with surgical blade or needle (for small blood quantities)
Amputation of the tail tip
Anterior vena cava
Dog and Cat: Cephalic (radial) vein, recurrent tarsal (saphenous) vein, jugular vein
Lab animals (rabbit, guinea pig, rats): cardiac puncture
Blood Quantity 🩸
Normovolaemia: Normal volume of blood (7-8% of body live weight)
Hypovolaemia: Haemoconcentration (higher haematocrit). Causes: diarrhea, sweating, bleeding
Hypervolaemia: Hydraemia (low haematocrit). Causes: liver disease (cirrhosis), kidney failure (loss of erythropoietin), heart insufficiency
Blood Color 🎨
Normal:
Venous: Dark red
Arterial: Bright red
Pathology:
Venous blood − pale: cyanide poisoning, leukaemia
Increase in CO2 (lungs, heart, bloating,...): dark
Nitrites poisoning − cherry-like
CO poisoning
Blood Coagulation = Haemostasis 🩸
A process causing bleeding to stop, meaning to keep blood within a damaged blood vessel (the opposite of haemostasis is hemorrhage). It is the first stage of wound healing; this involves blood changing from a liquid to a gel. Haemostasis also helps us to find out why a patient might have a bleeding disorder (hemorrhagic diathesis).
A. Bleeding Time
Small puncture wound
Swabbing away in 15–20 s intervals
Normal time: 3–5 minutes
Haemorrhagic diathesis: 10–20 minutes
B. Clotting Time
A drop of blood is placed on a transparent base (microscope or watch glass)
Every 30 s, a fine needle is inserted into the drop until threads of fibre begin to adhere
Normal time: 2–5 minutes
Erythrocyte Sedimentation Rate (ESR)
Measures how quickly RBCs settle at the bottom of a test tube
Normally, RBCs sink slowly, as it weights higher than that of plasma.
If they sink faster, it indicates inflammation in the body.
factors that increase ESR:
Low hematocrit and blood viscosity - fewer RBCs
high fibrinogen levels - in pregnancy, vascular and heart diseases
Macrocytic RBC - larger
high WBC count
Factors that decrease ESR:
high hematocrit - more RBCs
change in RBCs shape
high albumin levels
Sedimentation Rate ⏳
The Westergren tube is used to measure the sedimentation rate of blood.
1hr | 2hrs | 24hrs | |
|---|---|---|---|
Cattle | 12 | 26 | 109 mm |
(8-22) | (14-41) | (92-120) | |
Swine | 68 | 83 | 112 mm |
Dog | 20 | 32 | 63 |
Horse: vertical - 5 (3.7), 10 (14.7), 30 (69,3), 24H (126nm).
Anticoagulant Additives
additive | amount required for 10 ml blood | suitability | storage [hr] |
|---|---|---|---|
heparin | 1 - 2 mg | cytology <br> plasma | 16 |
disodium EDTA <br> (10 % solution) | 10 - 20 mg | cytology <br> plasma | 10 |
sodium citrate <br> - powder <br> - 3.8 % solution | 20 - 40 mg | cytology | 10 |
sodium oxalate | 20 - 40 mg | cytology | 10 |
Wintrob additive <br> (1.2 g ammonium oxalate <br> + 0.8 g sodium oxalate in <br> 100 ml of dist. water) | 1 ml | cytology | 10 |
Methods: Haematocrit ⚗
1. Routine Blood Count Methods
Packed Cell Volume (PCV) – proportion of volume erythrocyte to blood
MACRO (Wintrobe tubes)
Centrifugation for 30 min at 3000 rpm
MICRO (capillary tubes)
Centrifugation for 5 min at 5000 rpm
The blood sample should be continuously gently shaken every time before examination.
Macrohematocrit – Wintrobe tubes
animal | PCV [I/l] |
|---|---|
dog | 0,37 - 0,55 |
cat | 0,24 - 0,45 |
horse | 0,32 - 0,53 |
pigs | 0,38 - 0,42 |
cattle | 0,30 - 0,40 |
sheep | 0,27 - 0,40 |
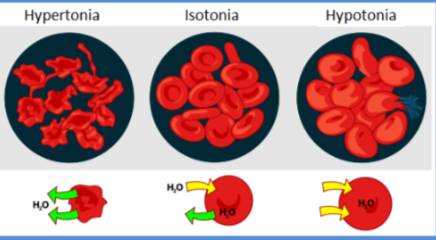
Normal Range & Pathological Changes
haemoconcentration - Increased PCV - dehydration, decreased water intake, decreased food intake.
Hydraemia - decreased PCV - bleeding, chronic disease (parasites), decreased erythrocyte size.
Haemoglobin 🩸
The iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates.
Methods
Sahli method
Colorimetric method (haemolysis)
Normal Range
animal | Hb [g/dl] |
|---|---|
dog | 12 – 18 |
cat | 8 – 14 |
horse | 11 – 17 |
pigs | 10 – 14 |
cattle | 9 – 12 |
sheep | 9 – 16 |
Pathological Changes
anemia
haemoglobinemia (free Hb in blood)
hemolysis, water poisoning, blood parasites, beet sugar leaves poisoning and decrease of P.
hemoglobinuria
Erythrocytes = Red Blood Cells 🔴
Erythropoietin: Inductor of erythropoiesis
Size: 4 – 8 µm
Life Span:
animal
days
dog
140 – 150
cat
80 – 120
horse
68 – 77
pigs
110 – 120
cattle
62 – 70
sheep
52
Non-mammals: Nucleated and elliptical
Mammals: Non-nucleated
Red Blood Cell Morphology
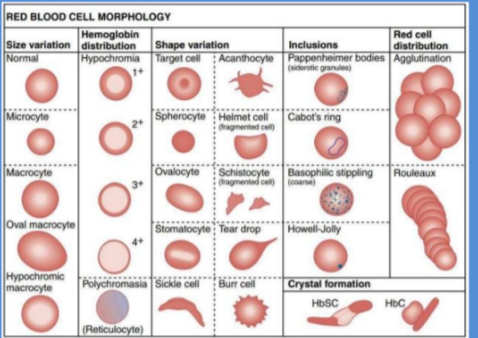
Erythrocyte Count
Microscopic Method
Objective 10
Hayem's diluting fluid (4975 μ l + 25 μ l blood)
Thoma blood celles diluting pipettes
Haemocytometer (Bürker's chamber) rule of the right angle
Normal Range
animal | [T/I; 10¹²/l] |
|---|---|
dog | 5 - 9 |
cat | 5 - 10 |
horse | 7 - 11 |
pig | 5 - 8 |
cattle | 5 - 10 |
sheep | 8 - 16 |
Erythrocyte Indices 📊
A. MCV (Mean Corpuscular Volume) [fI]
MCV=(PCV×1000)/RBCMCV=(PCV×1000)/RBC
Use: classification of anaemia
Normocytic
Microcytic
Macrocytic
B. MCH (Mean Corpuscular Haemoglobin) [fmol]
MCH=(Hb×6.207)/RBCMCH=(Hb×6.207)/RBC
Is the average mass of hemoglobin per red blood cell in a sample of blood
C. MCHC (Mean Corpuscular Haemoglobin Concentration) [mmol.l^-1]
MCHC=(Hb×0.6207)/PCVMCHC=(Hb×0.6207)/PCV
Use: classification of anemia
Normochromic
Hypochromic
Hyperchromic
Normal Range
animal | MCV [fl] | MCH [pg] | MCHC [g.dl⁻¹] |
|---|---|---|---|
dog | 52 – 70 | 12 – 16 | 31 – 36 |
cat | 40 – 55 | 7 – 11 | 26 – 34 |
horse | 40 – 49 | 6 – 10 | 31 – 37 |
pigs | 50 – 68 | 11 – 14 | 31 – 36 |
cattle | 30 – 56 | 9 – 15 | 26 – 34 |
sheep | 25 – 50 | 6 – 7 | 31 – 37 |
Erythrocyte Pathology
A. Shape
Poikilocytosis: Variation in shape
Degeneration
B. Size
Anisocytosis: Variation in size
Common feature in animals
Red blood cell distribution width (RDW) [%]
measure of variation of RBC volume reported as part of standard complete blood count.
RBCs - standard size of 6-8 μm in diameter.
Higher RDW: high variation in size - anisocytosis.
C. Number
Reduction: ANAEMIA
Polycythaemia:
Dehydration
Impairment of respiratory efficiency (heart, lungs diseases, erythropoietin)
Possible solution = Blood transfusion, which is generally the process of receiving blood products into circulation intravenously. Transfusions are used for various medical conditions to replace lost components of the blood.
Etiological Classification of Anaemia
a) Blood Loss Anaemia
Bleeding (ulcers, enteritis, coccidiosis, neoplasma haemophilia, vitamin C and K deficiencies)
Blood sucking parasites (endo, ecto)
Poisonings (warfarin)
Trauma, surgery
b) Haemolytic Anaemia
Infectious – Babesia, Anaplasma, Eperythrozoon (felis, suis), Clostridium haemolyticum
Toxic – copper, lead, mercury, rape, kale, water, furazolidon, incompatibe blood transfusion, beet sugar leaves
c) Dishaemopoietic Anaemia (Hypoplastic or Aplastic Anaemia)
Depression of erythrogenesis – anemia resulting from an inability of the bone marrow to produce red blood cells
Nutritional factors (vitamins B1, B2, B12)
Parasites
Hypothyroidism
Irradiation
Sulphonamides
d) Anemia of Chronic Disease
e) Hereditary Spherocytosis
f) Certain Hereditary Hemoglobinopathies
Including some cases of thalassemia minor
Feature of anaemia | Regenerative | Non-regenerative |
|---|---|---|
the major causes of regenerative anaemia are haemorrhage and haemolysis | the failure to regenerate indicates that there is a failure to produce red blood cells in the bone marrow | |
Mean Corpuscular Volume (MCV) | Increased as reticulocytes are larger than mature erythrocytes. | Normal |
Mean Corpuscular haemoglobin concentration (MCHC) | Decreased as reticulocytes are larger cells and less packed with haemoglobin (as the marrow is trying to produce cells at a faster rate it means they are not as well formed) and they also contain the remnant of the ribosomal RNA that is lost with progressive development of the cell. | Normal |
Blood Smear |
| The red blood cells are usually normochromic and normocytic but poikilocytosis may be apparent in cases of maturation defect anaemia. |
Symptoms of Anaemia
CNS: Fatigue, dizziness, fainting
Eyes: Yellowing
Skin: Paleness, coldness, yellowing
Blood Vessels: Low blood pressure
Heart: Palpitations, rapid heart rate, chest pain, angina, heart attack
Respiratory: Shortness of breath
Muscular: Weakness, recumbency
Intestinal: Changed stool color
Spleen: Enlargement
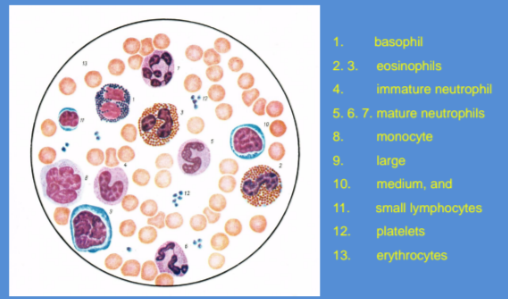
Leukocytes ⚪
Types
A. Polymorphonuclear cells (granulocytes) B. Mononuclear cells (agranulocytes)
Life Span
Lymphocytes: Few hours to a month
Granulocytes: Less than 14 days
Granulocytes
Neutrophils
Eosinophils
Basophils
Neutrophils characteristics:
10 – 15 µm
Ragged (mature) or smooth (immature) nuclear membrane
Acidophilic cytoplasm (light pink)
Pinkish granules
Deeply basophilic nucleus
Neutrophils are more or less regular in size across species, compared to RBC
Eosinophils characteristics:
10 – 14 µm
Pale-grey cytoplasm
Orange-red granules
Nucleus frequently non-segmented
Basophils characteristics:
12 – 22 µm
Purplish granules
Nucleus less intense stained
Lymphocyte Details (agranulocyte)
Lymphocytes can be small, intermediate, or large. In cattle, up to 50% can be intermediate. Here's a breakdown:
Nt-intermediate: Nucleus size is the same as Nt-large.
Nt can fit inside the nucleus (5-18 µm).
Blue narrow cytoplasm.
Excentric rounded dark blue nucleus (bluish granules – vacuoles).
Most are smaller than a neutrophil.
Lymphocytes are small, intermediate, or large. Small and mature lymphocytes are most common in peripheral blood.
In cattle, lymphocytes are in a 1:1 ratio with neutrophils.
Monocytes (agranulocyte)
12–20 µm (largest)
Variable shape nucleus
Net-like staining pattern
Factors produced at sites of inflammation can increase Mo production
White Blood Cell Functions 🔬
Here's a summary of the functions of different types of white blood cells:
Neutrophils
Phagocytosis of small particles.
Cellular defense mechanism (inflammation, bacteria).
Eosinophils
detoxification (histamine inactivation)
anti-parasitic defense mechanism
Basophils
Heparin and histamine release (increasing capillary permeability).
Lymphocytes
Cell-mediated immunity.
Immunoglobulin production.
Monocytes
Phagocytosis of large particles.
Wandering macrophage (tissues).
In leucocyte count - Giga per liter (G/l)
The blood sample in a sample tube with K3EDTA anticoagulant should be continuously and gently shaken before examination to preserve homogenous consistency.
Total Leukocyte Count 🔢
Microscopic - flask method:
Türk's diluting fluid (475 μl + 25 μl blood)
Sample tubes or Thoma blood cells diluting pipettes
Haemocytometer = Bürker's chamber
Here are the normal ranges for total leukocyte count:
Species | Range [G/l] |
|---|---|
Cattle | 6 - 10 |
Sheep | 5 - 11 |
Goats | 4 - 13 |
Pigs | 11 - 18 |
Horse | 6 - 9 |
Dog | 6 - 15 |
Cat | 5 - 11 |
Differential Leucocyte values 🩸
Obtained from stained blood smears.
Best smears are made on clean, grease-free, dry glass slides.
A small drop of blood is placed near the right-hand end of a horizontally situated glass slide.
Another slide called "spreader" (with shortened width by one-third) is held at a 30–45° angle.
The angled slide is pushed (spreader) to the left.
The dried blood smear is immediately stained with Leukodiff 200.
differential WBC count is gotten by counting 100-200 WBC, using the meander method and immersion lens of microscope.
Such stained blood film can be examined even for RBC parasites.
To obtain the percentage value of each cell type, make a written record.
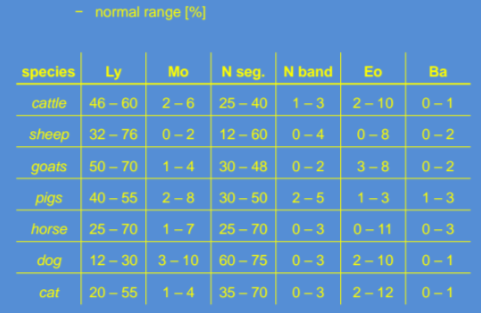
Here are the normal ranges for differential leukocyte values:
Common Counting Errors ❌
Improper lighting of the microscope field
Careless examination and enumeration of cells
Dirty equipment or diluting fluid
Poor staining technique (stain too light or too dark)
Improperly prepared blood films (too thick or too thin)
Leucocyte Pathology 🩺
Total WBC
Leucocytosis: ↑ number of leucocytes
Leucopenia: ↓ number of leucocytes (infectious feline enteritis)
Differential WBC
Neutrophils
Neutrophilia
Infective inflammatory processes
Stress
Hereditary defects in calves
Neutropenia
Earlier phases of viral infection (mouth foot disease, canine distemper, canine inf. hepatitis)
Lymphocytes
Lymphocytosis
Lymphopenia
Monocytes
Monocytosis
Feature of recovery
Protozoal infections
Chronic infections (brucellosis, Listeria mono.)
Eosinophils
Eosinophilia
Allergic conditions
Eosinophilic myositis
Parasitic diseases
Basophils
Basophilia: Parenteral introduction of heterologous
Allergic conditions
Protein
Mycosis
Decreased function of the thyroid gland
Sideropenic anemia
Left shift: ↑ immature forms (bad prognosis)
"Degenerative left shift": ↑ immature forms + total WBC normal or only slightly increased
Shift to the right: ↑ mature forms (inflammation)
Disease Progression Pattern 🔄
Early stages
Absolute neutrophilia (↑ immature forms)
↓ lymphocytes (often relatively)
Transient neutropenia (most marked in viral diseases)
Disease subsidence
↓ neutrophils
↑ lymphocytes and monocytes
Recovery
↑ lymphocytes and eosinophils
Gradual return to the normal leukocyte picture
In a proportion of infectious and other inflammatory diseases, there is a more or less regular pattern.
Thrombocytes (Platelets) 🩸
Platelets are produced in blood cell formation (thrombopoiesis) in bone marrow by budding off from megakaryocytes.
The physiological range for platelets is 150–400 G/l.
The lifespan of circulating platelets is 5 to 9 days.
Megakaryocyte and platelet production is regulated by thrombopoietin, a hormone usually produced by the liver and kidneys.
Old platelets are destroyed by phagocytosis in the spleen and by Kupffer cells in the liver.
Reserve platelets are stored in the spleen and are released when needed by sympathetically induced splenic contraction.
The function of platelets is the maintenance of hemostasis. This is achieved primarily by the formation of thrombi when damage to the endothelium of blood vessels occurs. On the converse, thrombus formation must be inhibited at times when there is no damage to the endothelium.
Electrical Impedance Methods (Blood Cell Analyzers) ⚡
This method counts blood cells by measuring how they change electrical resistance as they pass through a tiny opening. Cell size matters, as the size determines how the electrical resistance changes, allowing the machine to count and differentiate cells based on size.
Limited differentiation: due to its reliance only on size, it cannot distinugish between cells of the same size but with different internal structures. For ex. cannot tell difference of reticulocytes and erythrocytes, or band neutrophils from segmented.
Popular method: despite its limitations, its widely used method in hematology analyzers.
Only 3 WBC populations are counted: lymphocytes, monocytes and granulocytes.
Flow Cytometry Methods (Blood Cell Analyzers) 🔬
Considered the best method for the differentiation of cell populations.
Measures multiple characteristics of individual particles flowing in single file in a stream of fluid.
Light scattering at different angles can distinguish differences in size and internal complexity.
Light emitted from fluorescently labeled antibodies can identify a wide array of cell surface and cytoplasmic antigens.
This approach makes flow cytometry a powerful tool for detailed analysis of complex populations in a short period of time.
It cannot separate reticulocytes from erythrocytes or band neutrophils from segment neutrophils depending on their internal structure.
Dot plots are a visual representation of the complete blood count (CBC); each dot represents a single cell. Dot plots are a critical element of the CBC, providing a snapshot of cellular morphology.
Comparison of Blood Count Methods ⚖
Electrical Impedance
Small amount of blood.
Gives quick information (3 min) about blood count.
Depends only on the size of cells without their intracellular differentiation.
Can mistake small Ec for large Tc or small Lc, which can give incorrect results.
Pathological states in the blood picture must be checked by manual blood count.
In ruminants, manual differential WBC is necessary.
Flow Cytometry
Very expensive.
Small amount of blood.
One of the newest, most correct, and precise methods.
Can detect external and intracellular antigens of various characters.
Can differentiate cells by their size and granularity.
Gives complete differential WBC.
Can detect immature cells (band neutrophils and reticulocytes).
Routine Blood Count
The cheapest.
The slowest (45 min).
Requires skills and routine.
Manual dilution affects precision.
Requires a greater amount of blood, smear, and staining.
Differential WBC (30 min).
Examples of Changes in Leukograms 🐮
Dairy Cow, 1st Sampling, Diarrhea
Minimum RBC variation in size and shape.
Large central pale zone.
RBC fragments.
Rouleaux formation.
Small lymphocytes.
Segmented and band neutrophils.
Increased serum protein.
Dairy Cow, 2nd Sampling, Diarrhea
Varying RBC size.
Smaller central pale zone.
RBC fragments.
Acanthocytes.
Large and small dotted lymphocyte.
Hypersegmented large neutrophils.
Dairy Cow, 3rd Sampling, Diarrhea
Acanthocytes.
Dotted RBC with changed size.
Howell-Jolly’s bodies.
Missing central zone.
Segmented Nt, Eo, Ly, lymphoblasts.
Increased serum protein.
Calf, 1st Sampling, Chronic Bronchitis, Endoparasites
Changed RBC size and shape.
Varying central zone.
Mid-size Ly.
Segmented Nt.
Increased serum protein.
Platelets.
Calf, 2nd Sampling, Chronic Bronchitis, Endoparasites
Increased RBC density.
Changed RBC size and shape.
Large central zone and unclear margin.
Small Ly.
Segmented Nt.
Bull, 6 months old, Purulent Omphalophlebitis, Diarrhea
Acanthocytes.
Changes in RBC size.
Large immature RBC without central pale zone.
RBC fragments.
Large Eo.
Segmented Nt.
Small Ly.
Goat, 5 years old, Chronic Verminous Bronchopneumonia
Missing central pale zone.
Unclear cells.
Rouleaux formation.
Segmented and band Nt.
Eo.
Platelets.
Sheep, 4 years old, Necrobacillosis, Chronic Verminous Bronchopneumonia, Trichostrongyloidosis
Large central pale zone.
Changes in RBC size and shape.
RBC fragments.
Rouleaux formation.
Segmented Nt, Ly.
Platelets.