AP Bio Unit 3 Review
Enzyme Structure and Catalysis
Enzymes are proteins that speed up reactions by lowering activation energy.
They do not change the energy difference between reactants and products
Active sites bind substrates; specificity (the fact that enzymes are specific to their substrates) is key.
Factors affecting enzyme activity: temperature, pH, and substrate concentration.
Having sub optimal conditions for these factors can denature the enzyme
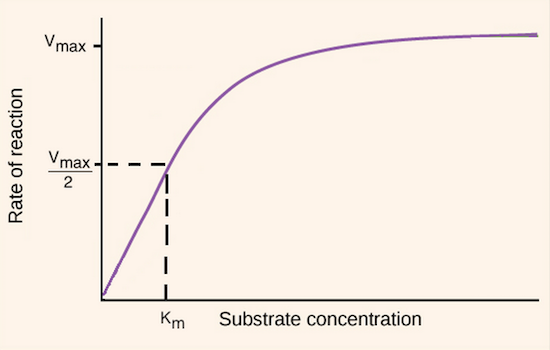
if the amount of enzymes stays constant, if you add so much substrate that all the enzymes are activated, activity can still plateau
High temperature - disrupts interactions b/t amino acids which can denature the enzyme which makes it lose secondary and tertiary structure
Enzymes may require coenzymes or cofactors to help catalyze reactions
Environmental Impacts on Enzyme Function
Temperature and pH can denature enzymes, altering their shape and function.
Inhibitors (competitive and non-competitive) can decrease enzyme activity.
Competitive inhibitors bind to the active site which compete with substrates
Noncompetitive inhibitors bind to an allosteric site which changes the active site
Regulatory molecules - Enzyme activity may be turned "up" or "down" by activator and inhibitor molecules that bind specifically to the enzyme.
Cofactors - Many enzymes are only active when bound to non-protein helper molecules known as cofactors.
Compartmentalization - Storing enzymes in specific compartments can keep them from doing damage or provide the right conditions for activity.
Feedback inhibition - Key metabolic enzymes are often inhibited by the end product of the pathway they control (prevents too much product from being made)
Cooperativity - When a substrate serves as an allosteric activator (binds to one site which increases activity of other sites)
Compartmentalization - Enzymes are stored in a specific part of the cell to do their job
Vmax means maximum velocity (rate of reaction)
Proteolytic enzymes - enzymes that break down proteins
Cellular Energy
ATP (adenosine triphosphate) is the primary energy carrier in cells.
Energy is released when ATP is hydrolyzed to ADP and inorganic phosphate.
First Law of Thermodynamics - energy cannot be created nor destroyed, only change form or transferred
Second Law of Thermodynamics - in every energy conversion some amount of useful energy is converted to unusable energy (commonly heat)
Transfer of heat increases entropy of the environment
Entropy - the measure of a system's thermal energy per unit temperature that is unavailable for doing useful work
Structure of ATP
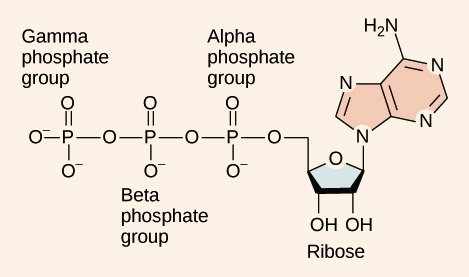
Phosphate group - A functional group characterized by a phosphorus atom bonded to four oxygen atoms
ATP Hydrolysis Reaction - ATP + H2O ←→ ADP + Pi + energy (Pi is inorganic phosphate group, and ATP regeneration is the opposite)
Reaction coupling - Energetically favorable reaction (ATP hydrolysis) is directly linked with an energetically unfavorable reaction (endergonic)
Shared intermediate - product of one reaction is “picked up“ and used as a reactant in a second reaction
Anabolic - building up complex molecule
Catabolic - breaking down complex molecule
Entropy: A measure of the disorder or randomness in a system. In thermodynamics, the entropy of the universe tends to increase.
Exergonic Reaction: A chemical reaction that releases energy, usually in the form of heat or work, and has a negative Gibbs free energy change.
Endergonic Reaction: A chemical reaction that absorbs energy, typically requiring an input of energy to proceed, with a positive Gibbs free energy change.
🌱 Photosynthesis
Occurs in chloroplasts; converts light energy into chemical energy (glucose).
Two Phases: (Light and Dark Reactions)
Light Reactions:
Sunlight is absorbed by chlorophyll, electrons are passed down a chain, and the energy molecules ATP and NADPH are produced. Water is also split into hydrogen and oxygen
Reactants: Light + Water (H₂O)
Products: Oxygen (O₂) + ATP + NADPH
Location: Thylakoid membranes
Process:
Photosystems are complexes of proteins and pigments that capture light energy.
Photosystem II (PSII):
First photosystem (named second in order of discovery).
Located in the thylakoid membrane.
Absorbs light energy (P680 chlorophyll a) to split water molecules into oxygen, protons, and electrons.
Excited electrons move through a series of proteins, creating a proton (H⁺) gradient that drives ATP synthesis via ATP synthase, similar to cellular respiration.
Photosystem I (PSI):
Also located in the thylakoid membrane.
Absorbs light energy to produce NADPH.
Peak absorption wavelength is due to chlorophyll a molecules (P700).
Excited electrons transfer to NADP⁺, reducing it to NADPH.
Dark Reactions (Light-Independent Reactions):
Sugar is made in the Calvin Cycle by using energy from ATP and NADPH, and the carbon from CO2
Location: Stroma of the chloroplasts
Process: Uses energy from ATP and NADPH to build sugars from carbon dioxide (CO₂)
Calvin Cycle (Light-Independent Reactions):
Reactants: ATP + NADPH + Carbon Dioxide (CO₂)
Products: Sugars
Location: Stroma
While the calvin cycle does not need light, it still needs the materials from light-dependent reactions, so photosynthesis still cannot occur at night in cactus plants
CO2 Contributes to most of the mass gain in plants, not water nor soil
Redox Reactions:
Oxidation: Loss of electrons
Reduction: Gain of electrons
Electron Transport Chain (ETC):
Electrons from Photosystem II (PSII) are transferred through the electron transport chain to Photosystem I (PSI).
The energy from these electrons is used to produce ATP and NADPH.
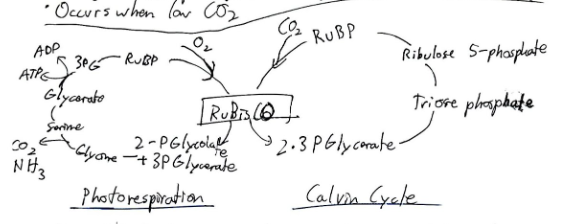
Calvin Cycle Steps:
Carbon Fixation
Reduction
Regeneration of RuBP
Overall Calvin cycle reaction:
Photorespiration:
C3 plants experience photorespiration, where the enzyme RuBisCO fixes oxygen instead of CO₂.
C4 plants and CAM plants have adapted mechanisms to minimize photorespiration.
Cellular Respiration
Process of breaking down glucose to produce ATP.
Stages:
Glycolysis (cytoplasm)
2 ATP used (investment)
4 ATP gained
2 NADH gained
Link reactions
Connects glycolysis with Krebs Cycle
Pyruvate moves into mitochondria
Reactants: 2 pyruvates and 2 NAD⁺
Produces:
2 Acetyl-CoA
2 CO₂
2 NADH
Krebs cycle (mitochondria)
Products: ATP, CO₂, NADH, and FADH₂
NADH and FADH₂ carry electrons to the electron transport chain (ETC)
Oxaloacetate is the starting and final compound
Electron Transport Chain mitochondria)
Series of proteins embedded in the inner mitochondrial membrane
Main site of ATP production
Requires oxygen (which is why you breathe)
Energy from electrons pumps H⁺ ions into the intermembrane space
ATP synthase controls the flow of H⁺ from high to low concentration (passively)
The flow of H⁺ produces ATP from ADP
Oxidative Phosphorylation: The production of ATP is coupled with the movement of electrons through the electron transport chain (ETC) via ATP synthase.
Electron Carriers:
NAD+ + H+ + 2 electrons = NADH
FAD + 2H+ + 2 electrons = FADH2
To carry electrons is to carry energy
Anaerobic respiration
occurs when cells lack oxygen to act as a final electron acceptor
pyruvates generated by glycolysis are broken down by fermentation to produce lactic acid or ethanol and NAD+
this permits glycolysis to continue and provide the 2 ATP it makes
Fitness
Relates to how efficiently organisms convert energy for growth, reproduction, and survival.
Metabolic rates and energy expenditure are key factors in fitness.
Key Terms:
Bioenergetics: The study of the flow and transformation of energy in living systems.
Redox Reaction: A chemical reaction involving the transfer of electrons between two substances, where one is oxidized (loses electrons) and the other is reduced (gains electrons).
First Law of Thermodynamics: Energy cannot be created or destroyed, only transferred or converted from one form to another (law of conservation of energy).
Second Law of Thermodynamics: The total entropy (disorder) of an isolated system always increases over time, and energy transformations are not 100% efficient.
Entropy: A measure of the disorder or randomness in a system. In thermodynamics, the entropy of the universe tends to increase.
Exergonic Reaction: A chemical reaction that releases energy, usually in the form of heat or work, and has a negative Gibbs free energy change.
Endergonic Reaction: A chemical reaction that absorbs energy, typically requiring an input of energy to proceed, with a positive Gibbs free energy change.
Energy Diagram: A graphical representation showing the energy changes that occur during a chemical reaction, often depicting the energy of reactants, products, and the activation energy.
Transition State: A high-energy, unstable state in a chemical reaction where old bonds are breaking, and new bonds are forming.
Activation Energy: The minimum energy required to start a chemical reaction, usually needed to break bonds in reactants.
Enzyme Specificity: The property of an enzyme to bind only to specific substrates, determined by the enzyme's active site structure.
Substrates: The reactants in an enzyme-catalyzed reaction that bind to the enzyme's active site.
Active Site: The region of an enzyme where the substrate binds and undergoes a chemical reaction.
Enzyme-Substrate Complex: A temporary complex formed when an enzyme binds to its substrate, facilitating the chemical reaction.
Induced-Fit: A model of enzyme action where the enzyme's active site undergoes a conformational change upon substrate binding to better fit the substrate.
Cofactors: Non-protein molecules or ions that are required for enzyme activity, either as a coenzyme or a metal ion.
Coenzymes: Organic molecules that act as cofactors, often derived from vitamins, and assist enzymes in catalyzing reactions.
Denatured: A process where an enzyme (or protein) loses its shape and, consequently, its function, usually due to extreme conditions like high temperature or pH.
Q10: A measure of the temperature sensitivity of a biological or chemical reaction, usually the factor by which the rate of reaction increases with a 10°C rise in temperature.
Allosteric Sites: Specific sites on an enzyme, other than the active site, where molecules (allosteric regulators) can bind and affect the enzyme’s activity.
Competitive Inhibition: A form of enzyme inhibition where a molecule competes with the substrate for binding to the active site of the enzyme.
Allosteric Inhibitor: A molecule that binds to the allosteric site of an enzyme and reduces its activity.
Noncompetitive Inhibition: A form of enzyme inhibition where an inhibitor binds to a site other than the active site, altering the enzyme's shape and decreasing its activity.
Light Reactions: The first stage of photosynthesis, where light energy is captured by chlorophyll and used to produce ATP and NADPH.
Dark Reactions: Also known as the Calvin Cycle, the second stage of photosynthesis, where carbon dioxide is fixed into organic molecules using ATP and NADPH produced in the light reactions.
Stroma: The fluid-filled space inside chloroplasts surrounding the thylakoid membranes, where the Calvin Cycle occurs.
Grana: Stacks of thylakoid membranes inside chloroplasts where the light reactions of photosynthesis take place.
Thylakoids: Membrane-bound structures within chloroplasts that contain chlorophyll and other pigments, and are the site of the light reactions of photosynthesis.
Chlorophyll a and b: The main pigments in plants that absorb light energy for photosynthesis, with chlorophyll a being the primary pigment and chlorophyll b assisting in light absorption.
Carotenoids: Accessory pigments in plants that absorb light energy and protect against damage from excess light, contributing to photosynthesis.
Reaction Center: The region of the photosystem where light energy is converted into chemical energy by exciting electrons.
Antenna Pigments: Pigments that absorb light and transfer the energy to the reaction center during photosynthesis.
Photosystem I: A protein-pigment complex involved in the light reactions of photosynthesis, primarily responsible for the production of NADPH.
Photosystem II: A protein-pigment complex involved in the light reactions, primarily responsible for splitting water molecules and producing ATP.
P680: The reaction center chlorophyll in Photosystem II, which absorbs light most efficiently at a wavelength of 680 nm.
P700: The reaction center chlorophyll in Photosystem I, which absorbs light most efficiently at a wavelength of 700 nm.
Photophosphorylation: The process of using light energy to generate ATP through the electron transport chain during the light reactions of photosynthesis.
Absorption Spectrum: A graph showing the wavelengths of light absorbed by a pigment or photosystem.
Emission Spectrum: The spectrum of light emitted by a substance after it absorbs energy.
Photolysis: The splitting of water molecules using light energy in the light reactions of photosynthesis, producing oxygen, protons, and electrons.
Carbon Fixation: The process of converting inorganic carbon dioxide into an organic molecule, primarily through the Calvin Cycle in photosynthesis.
Calvin-Benson Cycle: A cycle of reactions in photosynthesis where carbon dioxide is fixed into glucose and other organic molecules.
Photorespiration: A process that occurs when oxygen is incorporated into the Calvin Cycle instead of carbon dioxide, leading to a loss of energy and reduced efficiency in photosynthesis.
CAM Plants: Plants that use crassulacean acid metabolism to minimize water loss, opening their stomata at night to fix carbon dioxide.
C4 Plants: Plants that use a modified pathway for carbon fixation, where carbon dioxide is initially fixed into a 4-carbon compound, improving efficiency in hot environments.
Aerobic Respiration: Cellular respiration that occurs in the presence of oxygen, producing ATP by fully oxidizing glucose to carbon dioxide and water.
Anaerobic Respiration: Cellular respiration that occurs without oxygen, producing ATP by partially oxidizing glucose (e.g., fermentation).
Pyruvic Acid: A 3-carbon compound produced by the breakdown of glucose during glycolysis, which can be further processed in aerobic or anaerobic respiration.
Acetyl CoA: A 2-carbon molecule that is produced from pyruvic acid and enters the citric acid cycle in aerobic respiration.
Pyruvate Dehydrogenase Complex: An enzyme complex that converts pyruvate into acetyl CoA in preparation for the citric acid cycle.
Oxaloacetate: A 4-carbon molecule that combines with acetyl CoA to form citric acid in the citric acid cycle.
Citric Acid: A 6-carbon compound formed in the citric acid cycle from acetyl CoA and oxaloacetate.
Cytochrome C: A protein in the electron transport chain that transfers electrons between complexes and plays a key role in oxidative phosphorylation.
Proton Gradient: A concentration gradient of protons (H+) across a membrane, used in chemiosmosis to drive ATP synthesis.
Chemiosmosis: The process of generating ATP using a proton gradient across a membrane, as protons flow through ATP synthase.
ATP Synthase: An enzyme complex that synthesizes ATP from ADP and inorganic phosphate, using the energy of a proton gradient.
Oxidative Phosphorylation: The process of ATP production through the electron transport chain and chemiosmosis, occurring in the mitochondria.
Lactic Acid: A 3-carbon compound produced during anaerobic respiration in muscle cells, causing muscle fatigue.
Ethanol: A 2-carbon alcohol produced by fermentation in yeast and some bacteria.
Fermentation: An anaerobic process that allows cells to generate ATP by converting glucose into products like lactic acid or ethanol when oxygen is unavailable.