Chem Unit 8: Gas Laws
Gas General Properties
particles travel in straight lines
Collisions create pressure
fill containers uniformly and completely fast
Gases expand
BrINClHOF - diatomics
Volume -
Temperature - always in kelvin. When adding 273, its 3 sig figs. (adding sig figs is last decmial place) So when 30 + 273 = 303K. Use 3 sig figs when multiplying.
n- Moles
Pressure -
measured with a Barometer. Barometer pushes mercury up when pressure pushes down
Units: 1 atm = 760 mmhg/torr = 101.3 kPa
R Constant - 0.0821 (L)(atm)/ (mol)(K)
Gas Formulas
EVERY CALCULATION IN KELVIN
UNITS MUST BE THE SAME ON BOTH SIDES
When we are given STP sig figs don’t count. 0 C, 1 atm
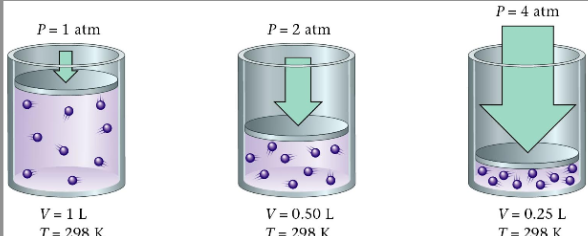
Boyles Law: P1⋅V1 = P2⋅V2
Inversely Related
when all other factors are constant (temp and moles stay the same)
If volume of the container increases, the pressure decreases. If the volume increases. If the volume of container decreases, the pressure increases.
if double the volume, the pressure 1/2. Opposite is true
Gay- Lussac Law: P1 / T1 = P2 / T2
Pressure and Temperature are directly related
If the temperature increases, the pressure increases.
MUST BE IN KELVIN.
if the temperature doubles in kelvin then the pressure doubles. Opposite is true
Charles Law: V1 / T1 = V1 / T1
Volume and Tempatuer are directly related
If the pressure is constant and there is a flexible container, the temperature will increase the collisions, increasing the volume. Example is balloon
Must be in Kelvin
Avagalros Law P/n
Adding a gas to a container increases the amount of particles that can collide, increasing pressure. This is a direct relationship. If the pressure exceeds, it will burst the container.
When removing
This is directly related to so if half of the moles are taken, half the pressure. The opposite is true
Combined Gas Law (P1⋅V1 )/T1 = (P2⋅V2) /T2
Use when Temp Change, Volume Change, Pressure Change. 5 Variables given.
Ideal Gas Law PV=nRT
Constant: R 0.0821 (L⋅ atm)/(Mol⋅K)
Gas Density
At STP 22.4/mol
D
Effusion/ Diffusion and Speed
Heaviest is slowest. Pay attention to Diatomic
Effusion is going out through a hole
Diffusion is high to low
Daltons Law: PTotal = PA+PB
Collecting Gas over water. (Total P = water vapor + Gas pressure)
Grahams Law
Altitude and Volume and Pressure: there is less pressure higher up so volume increases.
Grahams Law: Rate 1/Rate 2 = (Molar Mass 2 / Molar Mass 1)1/2
Rate of effusion is inversely proportional to its molar mass.
In terms of increasing: low to highest
in terms of decreasing: highest to low
REVIEW THE RATE AND MASS ONE