Carbon Chemistry Notes
Chapter 14: Carbon Chemistry
Elemental Carbon and Simple Carbon Compounds
Living organisms are composed of cells containing carbon, hydrogen, oxygen, nitrogen, and other elements.
Approximately 18% of the mass of living organisms consists of carbon compounds.
Most substances we interact with daily, such as food, clothes, cosmetics, and medicines, are carbon-based compounds (excluding water and some salts).
Organic Compounds
Historically, scientists believed that all carbon compounds originated from living or once-living organisms, terming them "organic".
Currently, it's recognized that carbon is present in many non-living entities as well.
An organic compound is defined as a chemical compound containing carbon atoms typically bonded to at least one hydrogen bond.
Types: hydrocarbons, alcohols, carboxylic acids, amides, amines
The Forms of Pure Carbon
Carbon atoms can bond in various arrangements, resulting in different forms such as graphite, diamond, fullerene, and amorphous carbon.
Graphite
In graphite, carbon atoms assemble into thin sheets that can slide or bend.
Diamonds
In diamonds, carbon atoms bond in an orderly, grid-like structure, providing extreme strength.
This arrangement makes diamond one of the hardest known materials.
Fullerene
Carbon atoms in fullerene form cage-like structures.
Fullerene was discovered late in the twentieth century.
Its uses are under investigation, with potential applications in developing faster, smaller electronic components.
Amorphous Carbon
The atoms in amorphous carbon lack an orderly arrangement.
Amorphous carbon is found in substances like soot, coal, and charcoal.
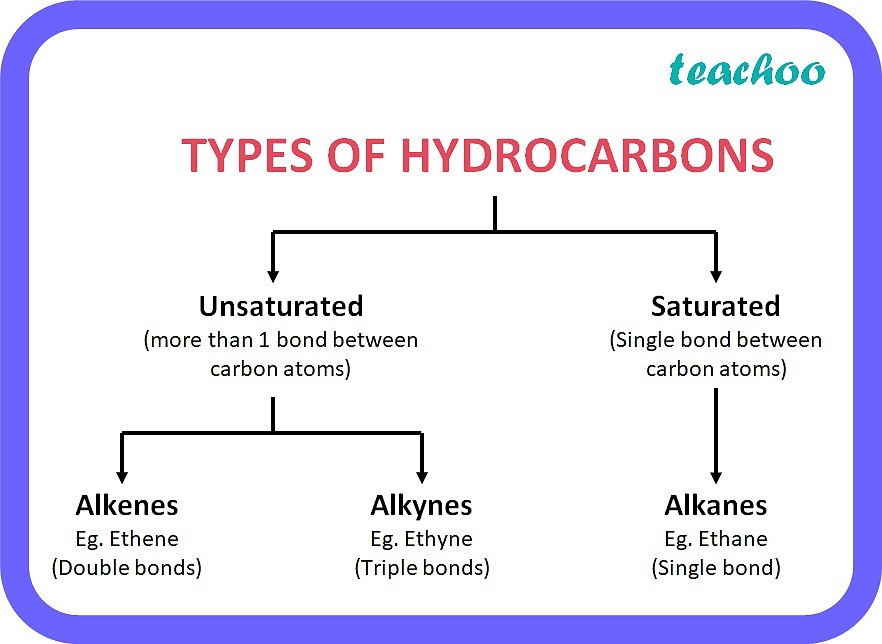
Hydrocarbons
Many organic compounds contain only carbon and hydrogen atoms; these compounds are called hydrocarbons.
Two types of hydrocarbons: saturated and unsaturated
Saturated Hydrocarbons
A hydrocarbon containing only single bonds is called a saturated hydrocarbon.
It's termed "saturated" because no more hydrogen atoms can be added to the molecule.
Unsaturated Hydrocarbons
A hydrocarbon containing one or more double or triple bonds is called an unsaturated hydrocarbon.
If the double or triple bonds are broken, additional hydrogen atoms can bond to the carbon atoms.
Naming Hydrocarbons
Examine the compound.
Count the number of carbon atoms in the longest continuous chain.
Determine the root name of the hydrocarbon.
Determine the type of bonds in the hydrocarbon.
Put the root and suffix together to name the hydrocarbon.
If the hydrocarbon is a ring, add -cyclo to the beginning of the name.