AP Bio Unit 3 Review Notes
Cellular Energetics: Unit 3
Gibbs Free Energy
Gibbs Free Energy is the energy available to do work.
When we discuss reactions in a cell, we consider whether they release or absorb energy. This relates to endergonic and exergonic reactions.
The Gibbs free energy equation is:
ΔG=ΔH−TΔS
Where:
ΔG = Change in Gibbs free energy (final minus initial)
ΔH = Change in enthalpy (heat energy)
TT = Temperature (in Kelvin)
ΔS = Change in entropy (disorder or chaos)
Also:
ΔG=Final Gibbs−Initial Gibbs
This helps determine if reactions are exergonic (negative ΔG) or endergonic (positive ΔG).
Endergonic Reactions
Energy must enter the system.
Start with low free energy and gain energy.
ΔG is positive (final G - initial G)
Products have more energy than reactants.
Non-spontaneous: Requires energy input to occur.
Absorbs energy.
Example: ADP + inorganic phosphate → ATP (requires energy input)
Exergonic Reactions
Energy exits the system.
Reactants have more free energy than products.
ΔG is negative (releases energy, final G - initial G).
Spontaneous: Will occur on its own, but may need activation energy.
Releases energy.
Example: ATP → ADP + inorganic phosphate + energy
Energy is not stored in the inorganic phosphate; it relates to the structural change.
Enzymes
Enzymes are biological catalysts that speed up chemical reactions within cells.
Reduce activation energy, which is the energy required to start a reaction.
Bind to molecules and put them in the appropriate conformation to react.
Can strain bonds to help break them.
ΔG remains the same.
Cannot change an endergonic reaction to exergonic, or vice versa.
Important Enzyme Properties
Property | Description |
|---|---|
Composition | Proteins (all protein concepts from unit 1 apply) |
Reaction Specificity | Enzymes can only catalyze very specific reactions because their active site can only bind very specific substrates. |
Not Consumed | Can be used repeatedly; only denaturing the enzyme will stop its function. |
Free Energy Effect | No effect on Gibbs free energy (ΔGΔG remains the same). |
Enzyme Function and Inhibition
How Enzymes Work
Enzymes function by lowering the activation energy of a reaction. They achieve this through an active site, where the substrate binds. Upon binding, the enzyme undergoes a conformational shape change, orienting the molecules appropriately for the reaction to occur.
Enzyme Reaction Process
Substrate binds to the active site.
Enzyme changes shape to properly orient the substrate.
Reaction occurs, converting the substrate into products.
Products are released.
Enzyme returns to its original state, ready for another substrate.
Inhibitors
Enzymes can be inhibited in different ways.
Competitive Inhibition
Competitive inhibitors compete for the same active site as the substrate. They often have a similar structure to the substrate.
A good analogy for competitive inhibitors is the relationship between morphine and endorphins. Morphine, a drug, has a similar structure to endorphins, natural pain relievers in the body, allowing it to bind to the same receptors and produce a similar effect.
Non-competitive Inhibition
Non-competitive inhibitors bind to an allosteric site, which is a different location on the enzyme than the active site.
When a non-competitive inhibitor binds, it causes the enzyme to change shape, which alters the active site and prevents the substrate from binding correctly.
Overcoming Inhibition
To overcome inhibition and increase reaction rate, you can:
Increase the amount of substrate.
Decrease the amount of product.
Slightly increase the temperature.
Increasing it too much will result in denaturing the enzyme
Product Inhibition
Sometimes the product of a reaction can act as an inhibitor. Because the product originally bound to the active site, it can bind again, inhibiting the enzyme.
Negative Inhibition
Denaturation
Denaturation occurs when the bonds in the secondary, tertiary, and quaternary structures of an enzyme break, causing the enzyme to lose its shape and function.
Factors Affecting Denaturation
Temperature: High temperatures increase kinetic energy, causing bonds to break.
pH: Changes in pH can cause the acidic carboxy groups and basic amino groups to donate or accept protons, altering the enzyme's shape. Enzymes have an optimal pH range for function.
Salinity: Changes in salinity can disrupt ionic bonding, leading to denaturation.
Cellular Respiration

Overview
Cellular respiration consists of three main steps:
Glycolysis
Krebs Cycle (Citric Acid Cycle)
Oxidative Phosphorylation
It is important to focus on what goes in, what comes out, where it takes place, and why it is important rather than trying to memorize every step and enzyme.
Glycolysis
Glycolysis takes place in the cytosol and does not require a mitochondria.
Input: Glucose (6-carbon structure)
Output: 2 NADH, 2 Pyruvate (3-carbon structures), 2 ATP
Glycolysis, meaning "sugar splitting," involves breaking glucose into two pyruvate molecules.
Pyruvate Oxidation / Prep Reactions
Pyruvate undergoes oxidation, resulting in the formation of acetyl CoA.
Process: Oxidation
Output: Acetyl CoA (2-carbon), 1 Carbon Dioxide, 1 NADH
During pyruvate oxidation, one carbon dioxide molecule is released.
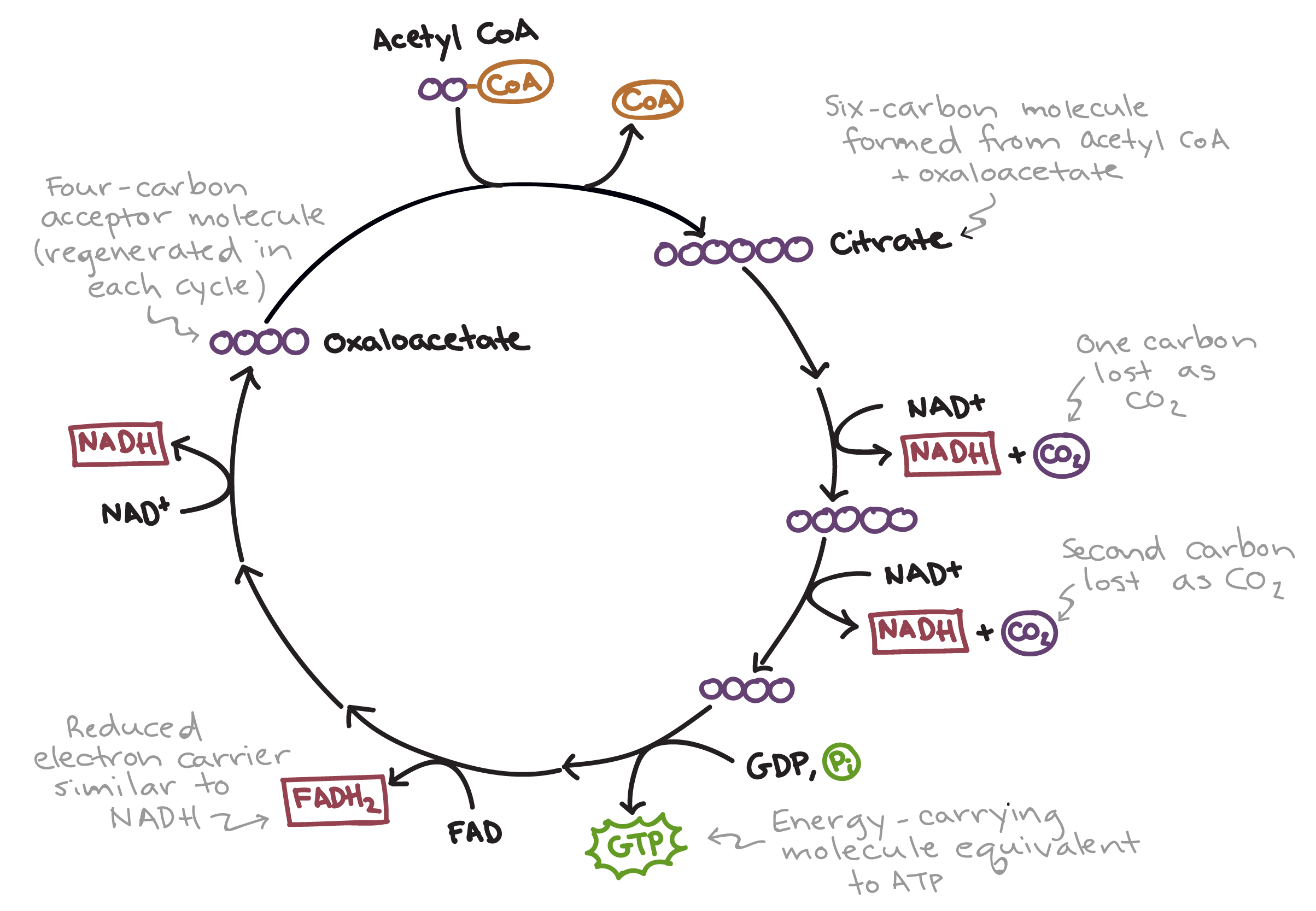
Krebs Cycle (Citric Acid Cycle)
The Krebs cycle takes place in the mitochondrial matrix, requiring a mitochondria.
Input: Acetyl CoA
In one turn of the Krebs cycle:
Output: 2 Carbon Dioxide, 3 NADH, 1 FADH2, 1 ATP (technically GTP)
For each glucose molecule, the cycle must run twice because glycolysis produces two pyruvate molecules, each yielding one acetyl CoA. By the end of the Krebs cycle, the original glucose molecule is completely broken down into carbon dioxide.
Oxidative Phosphorylation
In oxidative phosphorylation, the high-energy electrons from NADH and FADH2, produced during glycolysis and the Krebs cycle, are used to generate ATP. This process consists of two steps: the electron transport chain and chemiosmosis.
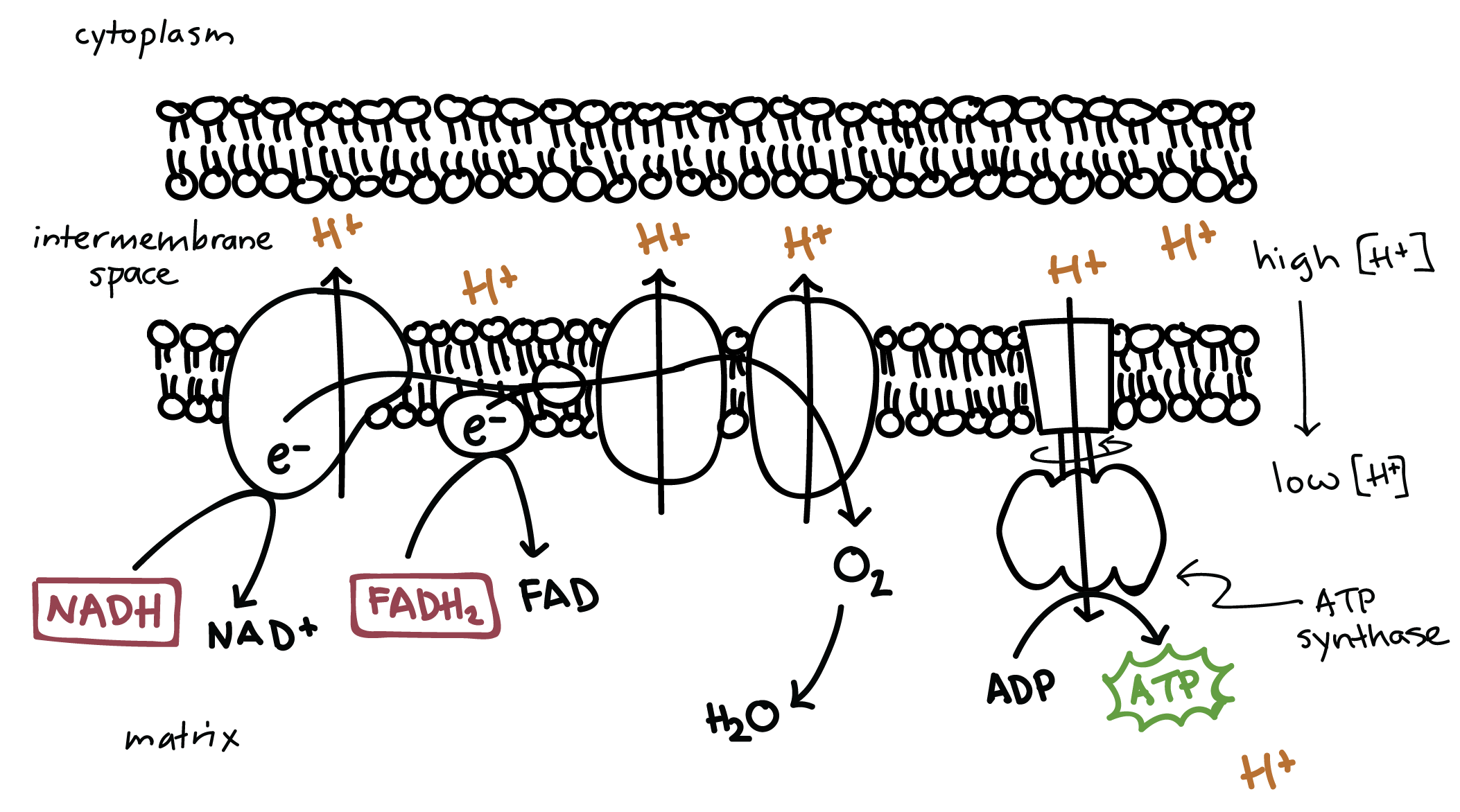
Electron Transport Chain
The electron transport chain (ETC) is located in the mitochondrial cristae, the highly folded inner membrane of the mitochondria.
NADH and FADH2 drop off electrons, which then move through the ETC.
As electrons move through the chain, they gradually lose energy.
This energy is used to pump protons (H+) from the mitochondrial matrix into the intermembrane space, creating a high concentration of protons in the intermembrane space and a low concentration in the mitochondrial matrix.
The intermembrane space becomes very acidic due to the high concentration of protons.
The intermembrane space (IM space) is the region between the inner and outer mitochondrial membranes.
Chemiosmosis
Chemiosmosis is the second step of oxidative phosphorylation.
The high concentration of protons in the intermembrane space creates a proton gradient.
Protons naturally flow down their concentration gradient, back into the mitochondrial matrix, through an enzyme called ATP synthase.
ATP synthase uses the potential energy stored in the proton gradient to phosphorylate ADP, generating ATP.
Inorganic phosphate (Pi) is used in the phosphorylation of ADP.
ATP synthase is an enzyme that synthesizes ATP using the energy from the proton gradient.
In summary:
The electron transport chain generates a proton gradient.
Chemiosmosis uses the proton gradient to produce ATP.
Cell Respiration Steps
Step | Location | Starts With | Importance |
|---|---|---|---|
Glycolysis | Cytosol | Glucose | Produces ATP via substrate-level phosphorylation, NADH, and acetyl CoA |
Krebs Cycle | Mitochondrial Matrix | Acetyl CoA | Produces ATP via substrate-level phosphorylation, NADH, and FADH2 |
Oxidative Phosphorylation | Cristae | Electrons from NADH and FADH2 | Creates a proton gradient, synthesizes ATP via chemiosmosis |
Photosynthesis
Photosynthesis consists of two main stages: light reactions and the Calvin cycle.
Light Reactions
The light reactions take place in the thylakoid membranes inside the chloroplasts.
Chloroplasts have an outer membrane, an inner membrane, and stacks of thylakoids called grana.
Thylakoid membranes contain chlorophyll, which absorbs light energy, giving plants their green color by reflecting green light.
The light reactions start with:
Water (H2O), which is broken down to release oxygen (O2) and electrons.
Photons of light energy
Process
Photosystem II (PSII) absorbs light energy.
Electrons move down an electron transport chain (ETC), and protons (H+) are pumped into the thylakoid space, creating a proton gradient.
The electrons then move to Photosystem I (PSI), where they gain more energy from light.
Finally, the electrons are transferred to NADP+, forming NADPH, which "holds" the electron during photosynthesis.
In summary:
Water is split, releasing oxygen.
Light energy is absorbed by chlorophyll.
A proton gradient is generated across the thylakoid membrane.
ATP and NADPH are produced.
Photosynthesis Products: ATP and NADPH
ATP and NADPH are produced due to the proton gradient.
In linear electron flow, Photosystem II, the electron transport chain, Photosystem I, and NADPH work together to synthesize ATP and NADPH in roughly equal amounts.
In cyclic electron flow, electrons from Photosystem I cycle back through the electron transport chain, enabling ATP production without NADPH synthesis.
This is important because the Calvin cycle requires nine ATP molecules but only six NADPH molecules.
Calvin Cycle
Occurs in the stroma of the chloroplast.
The stroma is the cytoplasm of the chloroplast
Utilizes three carbon dioxide molecules, nine ATP, and six NADPH.
Carbon dioxide binds to RuBP (ribulose-1,5-bisphosphate) with the help of the enzyme Rubisco.
The cycle produces G3P (glyceraldehyde-3-phosphate), a molecule also found in glycolysis.
Two G3P molecules can combine to form glucose.
RuBP must be regenerated.
Overall, carbon fixation, reduction, and regeneration are the key steps.
Input
Output
3 Carbon Dioxide
1 G3P
9 ATP
6 NADPH
C4 and CAM Plants
C3 Plants:
Under normal conditions, these plants break and make a three-carbon structure.
C4 Plants:
Important in arid conditions.
Use a four-carbon structure to initially fix carbon.
The stomata (pores in the leaf) close to conserve water, reducing carbon dioxide intake.
The closure of the stomata is regulated by guard cells.
C4 plants are an adaptation to these arid conditions.
CAM Plants:
Also found in arid conditions.
Open stomata at night to fix carbon into organic acids.
During the day, carbon is released from these organic molecules for use in photosynthesis while keeping the stomata closed to prevent water loss.
Example: Cactus.
Fitness in this context refers to the adaptation of individuals in warmer environments, modifying C3/C4/CAM pathways.
Key Concepts in the Calvin Cycle
Carbon Fixation: Carbon dioxide is bound/fixed.
Reduction: ATP and NADPH are used to add electrons to carbon.
Remember OIL RIG: Oxidation Is Loss, Reduction Is Gain (of electrons).
Rearrangement: Regeneration of RuBP.
Photosynthesis Chemical Reaction
The chemical reaction for photosynthesis: C6H12O6 + light energy → C6H12O6 + 6O2 + 6H2O
If the water input is labeled with a radioactive isotope of oxygen, the oxygen gas released comes from the water molecules split during the light reactions at Photosystem II.
Respiration Rates in Crickets and Mice
Endotherms (warm-blooded organisms like mice) regulate their body temperature through metabolism.
Ectotherms (cold-blooded organisms like crickets) rely on the environment to regulate their body temperature.
Mice at 10 degrees Celsius demonstrate greater oxygen consumption per gram of tissue compared to mice at 25 degrees Celsius.
Respiration Rates and ATP Production
If mice respirate more at 10°C than at 25°C, it indicates a higher respiration rate, implying more ATP is synthesized due to the increased frequency of cellular respiration.
A higher respiration rate means more ATP is being produced.
Skin Coloration and Melanin
Melanin is found in higher amounts in individuals living near the equator because it breaks down UV radiation, leading to darker complexions. Conversely, individuals living near the poles have lighter complexions due to less direct UV radiation.
Aerobic Respiration vs. Fermentation ️
Fermentation occurs when pyruvate can't enter the mitochondria or when mitochondria are absent. It regenerates NAD+ but produces less ATP compared to aerobic respiration.
Fermentation involves glycolysis followed by a process that regenerates NAD+ so that glycolysis can continue.
ATP Production Comparison
Process | ATP Produced |
|---|---|
Glycolysis (alone) | 2 |
Aerobic Respiration | 32-34 |
Aerobic respiration produces significantly more
per glucose molecule than fermentation.
Experimental Design: Negative Control
A negative control isolates the variable to determine if it's the actual cause of the observed effect. In this context, it helps determine if ATP production is due to the compound or the solvent used to dissolve it.
A suitable negative control would involve running the experiment without the compound to see the effect of the solvent alone (e.g., DMSO).
Impact of Glucose Availability
If there's no glucose, glycolysis cannot occur, and ATP production would halt. However, other molecules like fats and proteins can be broken down to enter the Krebs cycle, potentially leading to some ATP production.
Oxygen Consumption and Electron Transport Chain
If a compound stimulates the electron transport chain, oxygen consumption increases because oxygen is the final electron acceptor.
More electrons being transferred means more oxygen is needed to accept those electrons in the electron transport chain.
Photosynthesis Pathways
Non-Cyclic Electron Flow
Electrons pass from photosystem II, through the electron transport chain, to photosystem I, reducing NADP+ to NADPH.
Cyclic Electron Flow
Electrons cycle back to photosystem I and the electron transport chain instead of reducing NADP+.
Role of Chlorophyll
Chlorophyll absorbs solar radiation and is the site where water molecules are broken down to release electrons. It captures and absorbs light energy and receives electrons from water or the electron transport chain.
Cyclic Electron Flow
Electrons undergo electron transfer.
Electrons move into photosystem one.
NADPH to NADP+ Ratio
A high ratio of NADPH to NADP+ causes an increase in electron flow through the cyclic pathway.
Excess NADPH means there is less NADP+ available to accept electrons.
Less NADP+ to accept electrons causes electrons to pass to the cyclic pathway, or from ferredoxin to the cytochrome complex.