General Chemistry Semester 2 Final Review
highkey the flashcards 4 this are only vocab based so beware
Chapter 9 stoichiometry
SECTION 1
Composition stoichiometry- deals with the mass relationships of elements n compounds
Reaction stoichiometry- involves the mass relationships between reactants and products n a chemical reaction
Based on chemical equations and the law of conservation of mass
All reaction stoichiometry calculations start with a balanced chemical equation. This equation gives the relative numbers of moles of reactants and products
Problem Type 1: Given and Unknown quantities are amounts in moles
Problem Type 2: Given is an amount in moles and Unknown is a mass that is often expressed in grams
Problem Type 3: Given is a mass in grams and unknown is an amount in moles
Problem Type 4: Given is a mass in grams and unknown is a mass in grams
Mole ratio- is a conversion factor that relates the amounts in moles of any two substances involved in a chemical reaction
The molar mass is the conversion factor that relates the mass of a substance to the amount of moles of that substance
To solve reaction stoichiometry problems, you will need to determine molar masses using the periodic table
SECTION 2
Balanced equations give amounts of reactants and products under ideal conditions
Mole-to-gram calculations require two conversion factors
Amount of given substance (mol) → amount of unknown substance (mol) —> mass of unknown substance (g)
Multiple the known quantity, by the appropriate conversion factors
Gram-to-mole conversions require the molar mass of the given substance and the mole ratio
Mass of given substance (g) —> amount of given substance (mol) —-> amount of unknown substance (mol)
Mass-to-mass calculations use the mole rato and the molar masses of the given and unknown substances
Mass of given substance (g) —> amount of given substance (mol) —> amount of unknown substance (mol) —> mass of unknown substance (g)
SECTION 3
One reactant limits the product of a reaction
Limiting reactant- the reactant that limits the amount of the other reactant that can combine and the amount of product that can form in a chemical reaction.
Excess reactant- the substance that s not used up completely in a reaction
Comparing the actual and theoretical yields helps chemists determine the reaction’s efficiency
Theoretical yield- the maximum amount of product that can be produce from a given amount of reactant
Actual yield- measured amount of a product obtained from a reaction
Percentage yield- the rato of the actual yield to the theoretical yield, multiplied by 100
Percentage yield = actual yield theoretical yield x 100
Chapter 10 states of matter
SECTION 1: THE KINETIC MOLECULAR THEORY OF MATTER
Scientists developed kinetic molecular theory of matter to understand the behavior or atoms and molecules that make up matter
Kinetic Molecular Theory (KMT) : Particles of matter are always in motion
Explains properties of solids, liquids, and gases in terms of energy of particles and forces that act between them
KMT explains the constant motion of gas particles
Help understand behavior and physical properties of gases
Ideal gas: model provided by the theory; a hypothetical gas that perfectly fits all the assumptions of the KMT
Assumptions of the KMT:
Gases consist of large # of tiny particles that are far apart relative to their size
For the same number of particles, the ones of gases will occupy a volume that is 1000x greater than the volume in liquid or solid state
This is because molecules of gases are further apart
Volume occupied by a gas is empty space —> This is why gases have a lower density than liquids and solids
Makes gases to be easily compressed
Collisions between gas particles and between particles and container walls are elastic collisions
Elastic Collision: No net loss of total KE
KE is transferred between two particles during collisions and will remain the same as long as the temperature is constant
Collisions happen when gas particles travel in a straight line and collide each other or container walls
Gas particles are in continuous, rapid, random motion, therefore they possess KE
Gas particles move in all directions
KE of particles overcomes the attractive forces between them, except near the temperature at which gas condenses and becomes a liquid
No forces of attraction between gas particles
Similar to billiard balls, when gas molecules collide, they will bounce apart instead of sticking together
Temperature of a gas depends on the average KE of the gas particle
KE of particle is given by the equation: KE = ½ mv2
m = mass of particle, v = speed of particle
All particles of a specific gas have the same mass, their KE depend only on their speeds
If temperature increases, the average speeds and KE of gas particles increase and vice versa
Gases at same temperature have same average KE
At the same temperature, particles of lighter gas, such as hydrogen, will have higher speeds than heavier gas, such as oxygen
The KMT explains the physical properties of gases
Applies only to ideal gases
Do not actually exist but gases behave nearly ideally if pressure is not very high and temperature is not very low
Physical properties of gases:
Expansion
No definite shape or volume
Completely fill any container they are enclosed and take its shape
Gas can expand to fill anything that has a larger volume
These facts can be explained by the KMT: gas particles move rapidly in all directions (assumption 3), with no attraction between them (assumption 4)
Fluidity
Gas particles glide easily past one another because of no attractive forces (assumption 4)
The ability to flow makes both has gas and liquid to be considered as fluids
Low Density
The density of a substance in gaseous state at atm is 1/1000 the density of it in liquid of solid state
This is because the particles are so much farther apart in the gaseous state (assumption 1)
Compressibility
During compression, gas particles are brought closer together (initially very far apart)
Volume will decrease
When containers are full of under pressured gas, they can contain more than 100x as many particles of gas as the same size unpressurized containers could contain
Diffusion and Effusion
Even without even stirred, gases can spread out and mix with one another
Ex: If the stopper of a container of ammonia is removed, ammonia gas will mix up and spread out with the air in the room
Random and continuous motion of gas molecules (assumption 3) will carry them throughout space
This is called diffusion: Spontaneous mixing of particles of two substances by their random motion (spread out)
Gases diffuse readily into each other and mix together due to the rapid motion and empty space between molecules
Ex: If two different gases are in two separate flasks, they will keep moving in their own flasks as long as the stopcock is closed
But once you open the stopcock, molecules from both gases will diffuse continuously back and forth through the stopcock opening
Effusion: Gas particles pass through a tiny opening
Rate of effusion is directly proportional to the velocity —> Molecules of low mass effuse faster than molecules of high mass
Real gases do not behave according to the KMT
Particles of real gases occupy space and exert attractive forces on each other
Real gas: A gas that doesn’t behave completely according to the assumptions of KMT
When does a gas not behave like an ideal gas? When pressures are high and temperatures are low, gas particles will be closer together and their KE will be insufficient to overcome the attractive forces
Ex: In a car engine cylinder, gas molecules will expand to fill the cylinder, but when you exert pressure on them, they will move closer and reduce the volume
KMT is more true for gases whose particles have little attraction (weak intermolecular force)
Noble gases show ideal gas behavior over a wide range of temperatures and pressures, therefore particles of these gases are monatomic and nonpolar
No real gas perfectly matches the behavior of an ideal gas
But under specific conditions like low pressure and high temperature, the behavior of these gases will be very close to that of an ideal gas
The more polar the gas molecules are (such as NH3), the greater the attractive forces and the more different the gas will be from ideal gas behavior
Nonpolar gases won’t deviate as much from ideal behavior
Nonpolar monatomic gas will deviate less from ideal gas behavior compared to a nonpolar diatomic gas (weaker intermolecular forces due to simpler structure and smaller size) —> the simpler/smaller the molecules are, the more similar they behave to an ideal gas
SECTION 2: LIQUIDS
The Intermolecular forces of liquids determine their properties
A liquid can be described as a form of matter that has a definite volume and takes the shape of its container
Properties of liquids can be understood by applying the kinetic-molecular theory.
Particles in a liquid are in constant motion
Particles in a liquid are closer together than the particles in a gas are
Particles in a liquid are more effective than those between particles in a gas
Example of these forces are dipole-dipole, london dispersion, and hydrogen bonding
Liquids are more ordered than gases because of stronger intermolecular forces and the lower mobility of the liquid particles
Particles are not bound together in fixed positions but move about constantly
Fluid- a substance that can flow and therefore take the shape of its container
Relatively High Density
Most substances are only slight less dense in a liquid state than in a solid state
Water is one of the few substances that becomes less dense when it solidifies
At the same temperature and pressure, different liquids can differ greatly in density
Relative Incompressibility
When a liquid is compressed, usually its volume will decrease
In contrast a gas under pressure would have only about 1/1000 of its volume at normal atmospheric pressure.
Liquids are much less compressible than gases because liquid particles are more closely packed together. Like gases, liquids can transmit pressure equally in all directions
Ability to Diffuse
Gases diffuse and mix with other gas particles
Liquids also diffuse and mix with other liquids
Any liquid gradually diffuses throughout any other liquid in which it can dissolve
The constant, random motion of particles causes diffusion in liquids, as it does in gases
Diffusion is much slower in liquids than in gases because liquid particles are closer together.
The attractive forces between the particles of a liquid slow their movement
As the temperature of a liquid is increased, the diffusion occurs more rapidly
Reason is average kinetic energy, and therefore the average speed of the particles, is increased
Surface Tension
Surface Tension- a property common to all liquids, a force that tends to pull adjacent parts of a liquid’s surface together, thereby decreasing surface area to the smallest possible size
Results from the attractive forces between particles of a liquid
The higher the force of attraction, the higher the surface tension (VARIES DIRECTLY)
Water has a higher surface tension than most liquids
Due to the large part of the hydrogen bonds water molecules can form with each other
Molecules at the surface of the water are a special case
They form hydrogen bonds with other water molecules beneath them and beside them, but not with the molecules in the air above them
Surface water molecules get drawn together and toward the body of the liquid, creating a high surface tension
Surface tension causes liquid droplets to take on a spherical shape because a sphere has the smallest possible surface area for a given volume
Capillary Action- the attraction of the surface of a liquid to the surface of a solid, is a properly closely related to surface tension
A liquid will rise quite high in a very narrow tube and will wet the tube if a strong attraction exists between the liquid molecules and the molecules that make up the surface of the tube
Attraction tends to pull the liquid molecules upward along the surface and against the pull of gravity
Process continues until the attractive forces between the liquid molecules and the surface of the tube are balanced by the weight of the liquid
The same process is responsible for the concave liquid surface, called a meniscus, that forms in a test tube or graduated cylinder
Evaporation and Boiling
Vaporization- the process by which a liquid or solid changes to a gas
Evaporation- the process by which particles escape from the surface of a non boiling liquid and enter the gas state
Evaporation occurs because the particles of a liquid have different kinetic energies
Particles with higher-than-average energies move faster
Some surface particles with higher-than-average energies can overcome the intermolecular forces that bind them to the liquid
Evaporation is a crucial process in nature
Boiling is the change of a liquid to bubbles of vapor that appear throughout the liquid
Boiling differs from evaporation
Formation of Solids
When a liquid is cooled, the average energy of its particles decreases
If the energy is low enough, attractive forces pull the particles into an even more orderly arrangement
Substance then becomes a solid
The physical change of a liquid to a solid by remove of energy as heat is called freezing or solidification
SECTION 3: SOLIDS
The particles in a solid hold relatively fixed positions
Particles of a solid are more closely packed
Intermolecular forces are more effective
Interparticle attractive (dipole-dipole-, London dispersion, H-bonding) exert stronger effects in solids
Attractive forces hold particles in relatively fixed positions, with only vibrational movement around fixed points
Since motions of particles are restricted, solids are more ordered than liquids and gases
Ordered level Gas < Liquid < Solid (?)
2 types of solids:
Crystalline solids
Most solids consist of crystals
Crystal: A substance in which particles are arranged in an orderly, geometric, repeating pattern
Amorphous solid
Noncrystalline solids (such as glass and plastics)
Particles are arranged randomly
Definite Shape and Volume
Can maintain definite shape without a container
Crystalline solids are geometrically regular (even their fragments have geometric shapes that reflect internal structure)
Amorphous solids have definite shape but not geometric
Ex: Glass can be molded into any shape and if it’s shattered, the fragments can have different irregular shapes
Volume only changes slightly with a change in temperature or pressure
Definite volume because their particles are closely packed
Little empty space that particles can be compressed
Crystalline solids do not flow since their particles are held relatively in fixed positions
Definite Melting Point
Melting: Physical change of solid to liquid by addition of energy as heat
Melting point: Temperature at which a solid becomes a liquid
At this temperature, KE of particles within a solid will overcome the attractive forces holding them together
Particles can break out of their positions in crystalline solids, which have definite melting points
Amorphous solids don’t have definite melting point and therefore have the ability to overflow a range of temperatures
Amorphous solids are sometimes referred to as supercooled liquids, substances that retain liquid properties even at temperatures that they appear as solids
This is because particles in amorphous solids are arranged randomly, similar to particles in a liquid
However, unlike particles in a true liquid, particles in amorphous solids are not constantly changing their positions
High Density and Incompressibility
Substances are most dense in solid state
Solids are denser than liquids and gases
This is because the particles in a solid are more closely packed
What is the least dense solid? Solid hydrogen
It’s density is about 1/320 of the densest element, Os (Osmium)
Less compressible than liquids
Solids such as wood and cork, seem compressible but they are not
This is because they have pores that are filled with air. When these pores are exerted by intense pressure, they will compress but not the solid matter itself
Low Rate of Diffusion
Diffusion does occur in solids but not as fast as in liquids
Crystal particles are arranged in a 3-dimensional lattice
Crystal Structure: the total 3-dimensional arrangement of particles of a crystal
Unit Cell:The smallest portion of a crystal lattice that still shows the 3D pattern of the entire lattice
Crystal Lattice: A coordinate system that represents the arrangement of particles in a crystal
Contain many unit cells packed together
7 types of symmetry
Cubic
Tetragonal
Hexagonal
Trigonal
Orthorhombic
Monoclinic
Triclinic
Enables scientists to use shape to classify crystals
Binding Forces in Crystals
4 types of crystals
Ionic
Structure: positive & negative ions in a regular pattern (ions can be monatomic & polyatomic)
Typically form when metals from Group 1 & 2 combine with nonmetals/nonmetallic polyatomic ions in Group 16 & 17
Qualities: Hard, brittle, high melting points, good insulators, non conductors
Why? Strong binding forces between positive and negative ions
Covalent Network
Structure: atoms are covalently bonded to their closest neighbors
Qualities: Hard, Brittle, high melting points, nonconductors or semiconductors
Covalent bonding extends throughout networks that include many atoms
Examples: diamond - Cx, quartz - (SiO2)x, and silicon carbide - (SiC)x
Typically are oxides of transition metals
Examples above are basically giant molecules
**Subscript x shows that the component in the parenthesis extends forever and ever
Metallic
Structure: metal cations surrounded by a sea of delocalized valence electrons
Qualities: high electrical conductivity
Electrons are from metal atoms belonging to the whole crystal
Covalent Molecular
Structure : Covalently bonded molecules held by intermolecular forces
Qualities: Low melting point, easily vaporized, soft, and good insulators
Nonpolar covalent: weak london dispersion forces between bonds (H2, CH4, C6H6)
Polar covalent: stronger dispersion forces like dipole-dipole or even H-bonding (H20 & NH3)
forces holding molecules together are weaker than covalent chemical bonds between atoms
The particles in amorphous solids are not arranged in a regular pattern
Amorphous Solid: “Without shape” atoms are not arranged in a regular pattern
Examples: glass & plastic
Glass: made by cooling certain molten materials that prevents crystallization
Plastic: molded at high temperatures and pressures (used in structural materials)
Electronic devices: solar cells, copiers, laser printers, flat panels for computer monitors & screens
Emily
SECTION 4: CHANGES OF STATE
Substances in equilibrium change back and forth between states at equal speeds
Why do some liquid chemical substances (like rubbing alcohol) have an easily detected scent? Molecules at the liquid’s surface have enough energy to evaporate, overcoming the attraction of neighboring molecules
Phase: Any part of a system that has uniform composition and properties
Condensation:process by which gas turns into a liquid
Equilibrium: dynamic condition in which two opposing changes happen at equal rates in a closed system
If the temperature of a CLOSED liquid stays constant, so will the rate of evaporation
Example:
In a closed bottle of rubbing alcohol, high energy molecules from the liquid begin to evaporate
As evaporation continues as a constant rate, vapor molecules hit the cap of the bottle and begin to condense back into liquid
Once the rate of condensation and evaporation are equivalent, equilibrium has been reached
Equilibrium Vapor Pressure of a Liquid
Equilibrium Vapor Pressure: Pressure exerted by a vapor in equilibrium with its corresponding liquid at a given temperature
Kinetic Molecular Theory:Temperature increase → increase avg KE of liquid’s molecules → increase # of molecules able to evaporate → increase in molecules in vapor phase → increased equilibrium vapor pressure
Any substance w=at a given temperature has a specific equilibrium vapor pressure
Strong attractive forces of liquid = smaller percent of molecules able to evaporate regardless of temperature
Low percent of evaporation = low equilibrium vapor pressure
Volatile Liquids: Liquids that are quick to evaporate and have weak forces between particles (ether)
A liquid boils when it has absorbed enough energy to evaporate
Boiling: conversion of liquid to vapor with the liquid & at the surface
Boiling Point: temperature at which the equilibrium vapor pressure of the liquid equals the atmospheric pressure
All energy absorbed is used to evaporate the liquid
If the temp of the liquid increases, the equilibrium vapor pressure does 2
Pressure doesn’t change = temperature is constant
Pressure above the heated liquid increases = temp rises until vapor pressure equals the new pressure & liquid comes to boil once more
Vacuum Evaporator: causes boiling at temps that are lower than usual
Condensed & evaporated milk
Boils w/o scorching milk or sugars
Pressure Cooker: cooks food quickly due to its sealed top. The seal builds pressure over the surface of the boiling water inside, increasing the pressure & therefore the temp.
Normal boiling point - at 1 atm, 100 degrees celsius
Energy must be added continuously to keep the liquid boiling
Added energy doesn’t change liquid’s temperature
Needed to continue vaporization & is stored in vapor as PE
Molar Enthalpy of Vaporization: amount of heat needed to vaporize 1 mole of liquid at the liquid’s boiling point at constant pressure
Measures attraction between particles of the liquid
Stronger liquid molecule’s attraction → increase energy needed → high molar enthalpy of vaporization (MEV)
Water’s H-bonding causes high MEV & makes it a cooling agent
Water vapor escaping takes a lot of heat energy away with them
Freezing
Freezing: physical change of a liquid to a solid
Liquid→ Solid + heat energy
Freezing Point: temp at which th solid and liquid are in equilibrium at 1 atm/760 torr/101.3 kPa pressure
KE: particles of the liquid and solid have the same KE
PE: energy lost during freezing now stored in liquid
Decrease in energy → Solid state is more ordered than the liquid state → increase in particle order
Pure Crystalline Solids
Melting point and freezing point are the same
Nomatter atm or outside temperature = ice & liquid water will maintain a temperature of 0 degrees celsius
Only after the ice has all melted, the addition of energy will increase the temperature
Adding Energy → shifts equilibrium to the right → increases proportion of liquid to ice
A Solid’s Molar Enthalpy of Fusion: amount of energy required to melt 1 mole of solid at the solid’s melting point
Energy is absorbed → pulls particles apart → solid’s PE increases → decrease in particle order
Twins with MEV: attraction between solid particles dictates magnitude of the molar enthalpy of fusion
Sublimation and Deposition
Sublimation: Change of a solid directly to a gas
Ex: Dry Ice (solid CO2) and Iodine sublime at ordinary temperatures
Deposition: Gas directly into a solid
Ex: Formation of frost on a cold surface
Certain conditions warrant water existing in all 3 phases at the same time
Phase Diagram: Graph of pressure vs temperature that shows the conditions under which the phases of substances exist. Also reveals how the states of a system change with changing temperatures or pressures.
SECTION 5: WATER
The properties of water in all phases are determined by its structure.
Chemical composition: 2 Hydrogens, 1 Oxygen, connected by polar covalent bonds in both ice and liquid states
The number of hydrogen bonds between molecules decreases with rising temperature due to increased kinetic energy
Liquid water: each molecule typically links with 4 to 8 other water molecules, stabilizing its liquid form
Nonpolar molecules do not form hydrogen bonds and primarily exist as gases at room temperature
Ice features a hexagonal molecular arrangement, leading to a lower density compared to liquid water due to the empty spaces between the molecules (this structure is a result of hydrogen bonding)
Ice melts -> water molecules gain energy, causing them to move closer together, This results in an increase in the density of water compared to ice
Water molecules are most tightly compacted at approximately 3.98°C (highest density)
As the temperature rises above this point, the kinetic energy energy of the molecule increases, causing them to move apart and result in a decrease in density
Water freezes at 0°C, boils at 100°C (1 atm pressure)
The molar enthalpy of water determines many of its physical characteristics.
The molar enthalpy of fusion (energy required to melt ice) is 6.009 kJ/mol, while the molar enthalpy of vaporization (energy required to convert water to vapor) is 40.79 kJ/mol
Both are relatively high due to the strong hydrogen bonding that must be overcome during these phase changes
Ice’s ability to float on liquid water is due to its lower density or else it would sink to the bottom of lakes and be less likely to melt
Both the boiling point and the molar enthalpy of vaporization of water are high compared to those of nonpolar substances of comparable molecular mass
The values are high because of the strong hydrogen bonding that must be overcome for boiling to occur
This high molar enthalpy of vaporization makes water useful for household steam heating systems (steams store a great deal of energy as heat) and in living organisms, it helps them resist dehydration
Gases and pressure
Collisions of air molecules generate pressure
If you pump air into an automobile tire, the pressure in the tire will increase
Pressure increase is caused by the increase in the number of collisions of molecules of air with the inside walls of the tire
The collisions cause an outward push, or force, against the inside walls.
Gas molecules exert pressure on any surface with which they collide.
The pressure exerted by a gas depends on volume, temperature, and the number of molecules present
Pressure (P) is defined as the force per unit area on a surface
Pressure = ForceArea
Pressure depends on Force and Area
SI unit for force is the newton, (N)
The newton is the force that will increase the speed of a one-kilogram mass by one meter per second each second that the force is applied.
At Earth’s surface, gravity has an acceleration of 9.8 m/s²
Example: A mass of 51 kg exerts a force of 500 N (51 kg x 9.8m/s²) on Earth’s surface. No matter how the dancer stands, she exerts the same force -500 N- against the floor. But the pressure exerted against the floor depends on the area of contact.
Atmospheric Pressure
The atmosphere, the shell of air surrounding Earth– exerts pressure.
The pressure of the atmosphere can be thought of as caused by the weight of the gases that compose the atmosphere.
Atmospheric pressure is the sum of the individual pressures of the various gases (nitrogen, oxygen, other gases) in the atmosphere
The Barometer
A barometer is a device used to measure atmospheric pressure
First introduced by Evangelista Torricelli
Wondered why water pumps could raise water to a maximum height of only about 34 feet
Though the height must depend somehow on the weight of water compared with the weight of air.
Reasoned that liquid mercy, about 14 times as dense as water, could be raised only 1/14 as high as water
How a Barometer Works
The space above the mercury in the tube of a barometer is nearly a vacuum
The mercury in the tube pushes downward because of gravitational force
Column of mercury is stopped from falling beyond a certain point because the atmosphere exerts a pressure on the surface of the mercury outside the tube.
This pressure is transmitted through the fluid mercury and is exerted upward on the column of mercury
The exact height of the mercury in the tube depends on the atmospheric pressure, or force per unit area.
Pressure is measured in terms of the mercury column’s height in the barometer tube
The atmospheric pressure at any given place depends on the elevation and the weather conditions
If the atmospheric pressure is greater than the average at sea level, the height of the mercury column in a barometer will be greater than 760 mm. (vise versa)
Using a Manometer
All gases exert pressure
Device called a manometer can be used to measure the pressure of an enclosed gas sample
EX: consider a can filled with air. The atmosphere exerts a pressure on the can. The air inside the can pushes outward and balances the atmosphere’s inward-pushing force. If a vacuum pump removes the air from the can, the outward force is removed, and the atmospheric pressure crushes the can.
Units of Pressure
A number of different units are used to measure pressure.
Atmospheric pressure is often measured by a mercury barometer, pressure can be expressed in terms of the height of a mercury column
Thus the common unit of pressure is millimeters of mercury, symbolized mm Hg.
1 mm Hg is also called 1 torr to honor Torricelli for his invention of the barometer
The average atmospheric pressure at sea level at O degrees Celsius is 760 mm Hg.
One atmosphere of pressure (atm) is defined as being exactly equivalent to 760 mm Hg.
In SI, pressure is expressed in derived units called pascals.
One Pascal (Pa) is defined as the pressure exerted by a force of one newton (1N) acting on an area of one square meter
1 atm is equal to 1.01325 x 10⁵ Pa, or 101.325kPa
Standard Temperature and Pressure
To compare gas volumes, one must know the temperature and pressure at which they are measured
For purposes of comparison, scientists have agreed on standard conditions of 1 atm pressure and 0 degrees celsius
These conditions are called standard temperature and pressure, STP
Pascal (Pa) 1 Pa = 1 Nm²
Millimeters of mercury (mm Hg)
Torr (torr) 1 torr = 1 mm Hg
Atmosphere (atm) 1 atm = 760 mmHg; 760 torr, 1.01325 x 10⁵ Pa, 101.325kPa
Pounds per square inch (psi) 1 psi = 6.89286 x 10³ Pa; 1 atm = 14.700 psi
SINCE THESE ARE EXACT DEFINITIONS OF MEASUREMENT THEY HAVE AN INFINITE NUMBER OF SIG FIGS
EX: P of atmosphere = 0.830 atm
760 mmHg = 1 atm (definition); 101.325 kPa= 1 atm (definition)
1 atm x 760 mm Hg1 atm= mm Hg
1 atm x 101.325 kPa1 atm= kPa
P= 0.830 atm x 760 mm Hg1 atm= 631 mm Hg
P= 0.830 atm x 101.325 kPa1 atm= 84.1 kPa
The total pressure of a gas mixture is the sum of the pressures of the gases in it
John Dalton, found that the pressure exerted by each gas in an unreactive mixture is independent of that exerted by other gases present
The pressure of each gas in a mixture is called the partial pressure of that gas
Dalton’s law of partial pressures states that the total pressure of a gas mixture is the sum of the partial pressures of the component gases
Dalton’s Law and the Kinetic-Molecular Theory
Each of the rapidly moving particles of gases in a mixture has an equal chance to collide with the container walls
Therefore each gas exerts a pressure independent of that exerted by the other gases present
Total pressure is the result of the total number of collisions per unit of wall area in a given time
Vapor Pressure of Water
Gas produced by the reaction displaces water, which is more dense, in the collection bottle.
You need to apply Dalton’s law of partial pressures in calculating the pressures of gases collected in this way
A gas collected by water displacement is not pure but always mixed with water vapor
Because water molecules at the liquid surface evaporate and mix with the gas molecules
In a closed system, water vapor, like other gases, exerts a pressure known as vapor pressure
EX: you wished to determine the total pressure of the gas and water vapor inside a collection bottle. You would raise the bottle until the water levels inside and outside the bottle were the same. At that point, the total pressure inside the bottle would be the same as the atmospheric pressure, Patm from a barometer in the laboratory. Subtract the vapor pressure of the water at the given temperature from the total pressure. The vapor pressure of the water varies with the temperature.
The Gas Laws
Pham
Gas Laws: relationships among the volume, temperature, pressure, and amount of a gas
Gas volume and pressure are indirectly proportional
Robert Boyle: doubling the pressure of gas at constant temperature = reducing its volume by ½
As pressure increases, volume decreases
Pressure of gas is caused by moving molecules hitting the container walls
Suppose the volume of a container decreases, but at the same temperature, the number of molecules stays the same —> More molecules per unit volume
As a result, number of collisions increases —> Pressure increases
Graphing Boyle’s Law
Boyle’s law: The volume of a fixed mass of gas varies inversely with the pressure at constant temperature
Boy’s law can be expressed as: PV = k
Since P and V vary inversely, their product remains the same
Inverse relationship between changes of pressure and volume can be expressed as: P1V1 =P2V2
P1 and V1 are the initial conditions while P2 and V2 are a different set of conditions
Gas volume and temperature are directly related
If pressure is constant, gases expand when heated
When temperature increases, the volume must increase since molecules collide with the walls of container more frequently with stronger force if the pressure is to stay constant
At higher temperatures, gas molecules move faster
Charles discovered that all gases expand to the same extent when heated through the same temperature interval
At constant pressure and initial temperature of 0 degree C, the volume changes by 1/273 of the original volume for each Celsius degree
Ex: Raising temperature from 0 to 1 degree C =increasing gas volume by 1/273
Same change occurs if a gas is cooled at constant pressure
Absolute zero : -273.15 degrees C or 0 in the Kelvin schlage
K = 273.15 + degree C
Charles’s Law
Relationship between temperature in K and gas volume
Volume of a gas at constant temperature varies directly with the temperature in K
Expressed as: V = kt or V/T = k
T = Temp. in K
k = constant
Ratio V/T always = k
Charle’s law equation can be applied to volume-temperature problems: V1/T1 =V2/T2
Gas pressure and temperature are directly related
Energy and frequency of collisions depend on KE of molecules
For a fixed quantity of gas at constant volume, pressure is directly proportional to temperature in Kelvins, which depends directly on avg KE
For every K of temperature change, the pressure changes by 1/273
Gay-Lussac’s law: Pressure of a gas at constant volume varies directly with the temp. in K
Expressed as: P = kt or P/T = k
T = temp. in K
k = constant; depends on quantity of gas and volume
For a given mass of gas at constant volume, ratio P/T is the same
Formula: P1/T1 = P2/T2
Gas, pressure, temperature, and volume are interrelated
When a gas undergoes changes in temp, pressure, and volume all at once, we combine all the three laws
Combined gas law: Expresses relationship between pressure, volume, and temperature of a fixed amount of gas
Expressed as: PV/T = k
Formula: P1V1/T1 = P2V2/T2
Indicates two different sets of conditions
T = temp in K
Each gas law can be obtained from combined gas law when the proper variable is constant
Boyle’s law: Temp. is constant
Charles’s law: Pressure is constant
Gay-Lussac’s law: Volume is constant
Gas Volumes and Ideal Gas LAw
Boyle’s Law
As pressure goes up, volume goes down = INVERSE RELATIONSHIP
Ex: es you decrease the pressure on a spacesuit, it EXPANDS
P1V1=600
P2V2=600
P1V1=P2V2
Charles's Law
As temperature increases, volume increases = DIRECT RELATIONSHIP
At -273 degrees celsius would theoretically 0 volume
V1/T1 = K (constant)
V2/T2 = K (constant)
V1/T1=V2/T2
Molecules move more quickly
Takes up more space
Collides with the wall more frequently
Steps to solving a boyle’s law equation
P1V1=P2V2
P1 = first pressure you see
V1= the first volume you see in the problem
P2 = 2nd pressure you see
V2= 2nd volume you see
** Note that one of these variables will likely be missing and you’ll have 2 solve for it
If you see the volume going up, expect the volume to go down & vice versa
Example:
P1V1=P2V2
P1=720 torr
V1=450 mL
P2=760 torr.
V2=x
720*450=760x
324000=760x
X = 426.3157895
Rounded to 2 sig figs - X = 430
Boyle’s Law/Charles’ Law Worksheet Answers
231 mL
Gay-Lussac’s Law
Studied gas volume relationships involving chemical reactions between hydrogen and oxygen
2L of H2(g) + 1L of O2(g) → 2L of water vapor
Gas reactions display a 2:1:2 volume ratio applicable to any proportions for volume
Constant pressure & temperature = volumes expressed as ratios of small whole #s
Different from Dalton’s theory that atoms are indivisible as O2 would have had to be split up to create a 2:1:2 volume ratio.
Equal volumes under the same conditions contain equal # of molecules
Avogadro’s Contributions
Established that there were 2x as many hydrogens than oxygens in H2O
All elements that are gases near room temperature (except noble gases) are diatomic
Used coefficients to balance equations
Avogadro’s Law
Same volume, temperature, and pressure = equal numbers of molecules
Volume varies directly with number of molecules
For Avo’s law to work, “k” must be a constant
Formula: V=kn
** note: “n” indicates the amount of gas
Standard Molar Volume of a Gas: 1 mol/ 22.4 L
Same volume, temperature, and pressure Does Not = same mass
Diffusion and Effusion
Diffusion - gradual mixing of two or more gases due to their spontaneous, random motion
Effusion - process where the molecules of a gas confined in a container randomly pass through a tiny opening in the container
Rates of diffusion and effusion depend on the relative velocities of gas molecules
Velocity of gas varies inversely with square root of molar mass
At same temp, lighter molecules move faster than heavier ones
Average kinetic energy depends on temperature and equals 1/2mv^2
Two different gases (A and B) at same temperature - ½ MAvA^2 = ½MBvB^2
Rate of effusion between two different gases at the same conditions (also can be used to find molar mass of unknown gas) -
Graham’s law of effusion states that the rates of effusion of gases at the same temperature and pressure are inversely proportional to the square roots of their molar masses.
TYPES OF MIXTURES
Solutions are homogeneous mixtures
By soluble we mean capable of being dissolved
A solution is a homogenous mixture of two or more substances uniformly dispersed throughout a single phase
Components of Solutions
In a solution, atoms, molecules, or ions are thoroughly mixed, resulting in a mixture that has the same composition and properties throughout.
The dissolving medium in a solution is called the solvent
The substance dissolved in a solution is called the solute.
The solute is generally designated as that component of a solution that is of lesser quantity
In a solution, dissolved solute particles are so small that they cannot be seen
Remain mixed with solvent indefinitely as long as the existing conditions remain unchanged
Types of Solutions
Solutions may exist as gases, liquids, or solids.
Many alloys, such as brass, and sterling silver, are solid solutions in which the atoms of two or more metals are uniformly mixed.
The Particles in a Suspension are Large
If the particles in a solvent are so large that they settle out unless the mixture is constantly stirred or agitated, the mixture is called a suspension.
Jar of muddy water, if left undisturbed, particles of soil collect on the bottom of the jar
Soil particles are denser than the solvent, water.
Particles in suspension can be separated from heterogenous mixtures by passing the mixture through a filter
Colloids have Particles of Intermediate Size
Particles that are intermediate in size between those in solutions and suspensions form mixtures known as colloidal dispersions, or simply colloids
After large soil particles settle out of muddy water, the water is still cloudy because colloidal particles remain dispersed in the water
If cloudy mixture is poured through a filter, the colloidal particles will pass through the filter, and the mixture will remain cloudy.
Particles in a colloid are small enough to be suspended throughout the solvent by the constant movement of the surrounding molecules.
Colloidal particles make up the dispersed phase, and water is the dispersing medium.
EX of Colloids: milk, hair spray, and photographic film
Colloids can be classified according to their dispersed phase and dispersed medium.
Different types of colloids have common names you may recognize
Emulsion is a liquid in a liquid, like milk
Clouds and fog, liquids dispersed in gas, are liquid aerosols
THE SOLUTION PROCESS
PG.394 Answers: hh
Factors that Affect Dissolving Between a Solid Solute and Liquid Solvent
Temperature
Increasing the temperature increases dissolution. An increase in temperature means an increase in kinetic energy, making the liquid solvent molecules move faster. This makes collisions between solid solute and liquid solvent molecules more frequent, increasing dissolution.
Ex: sugar dissolves quicker in hot tea than iced tea because the hot tea (solvent) has more kinetic energy and thus collides with the sugar (solute) more frequently.
How much temperature affects the solubility of a solid solute in a liquid is UNPREDICTABLE, more unpredictable than temperature’s effect on gas solubility.
Sometimes it affects the solubility a lot and sometimes very minimally
KNO3 in water solubility increases drastically with heat
NaCl in water solubility barely increases with heat
Surface Area of Solute
Ions and molecules of the solute are both attracted to the solvent molecules, causing them to leave the surface on the solid.
Since dissolution occurs on the face of the solute, the more surface area of the solute means more places for the solute’s molecules to break off of.
Ex: powdered sugar dissolves quicker than gradual sugar in water
Addition of Agitation
Mixing, stirring, or shaking the solution disperses the solute particles.
This makes them more spread out with more opportunities to mingle with the solute.
Ex: Ur friend group disperses during a party to bag the baddies faster than waiting for them to come up to your group
Solubility: How well one substance dissolves in another
Limits to the amount of solute that can dissolve in a solvent is dependent on the nature of the solute, nature of the solvent and the temperature.
Unsaturated Solution: A solution that contains less solute than a saturated solution under the existing conditions
Saturated Solution: A solution that contains the maximum amount of dissolved solute
Once the dissolution of the solute and recrystallization of the solute reaches equilibrium, it won’t be able to dissolve anymore solute. That solute is just excess now.
Ex: finding a perfect friend group makes you comfy. You don’t need anymore friends now.
Ex: To prepare a saturated solution of sugar in water, one would continuously add sugar into the water until no more sugar is able to be dissolved and sinks to the bottom of the container.
Supersaturated Solution: A solution that contains more dissolved solute than a saturated solution contains under the same conditions
Ex: To prepare a supersaturated solution of sugar water, take a saturated solution of sugar water and heat it up until the excess sugar dissolves into the water and let it cool devoid of disturbances.
“Like Dissolves Like”
Polarity and intermolecular forces affect the formation of ionic compounds.
Negatively charged Oxygen in water attracts the positive component of the ionic compound while the positively charged H2 in water attracts the negative component of the ionic compound
Ex: It’s more effective to use toluene, a nonpolar substance to remove lubricating oil (another nonpolar substance) on your clothing than water (polar). The nonpolar substances will dissolve each other, adhering to the principle that “like dissolves like”.
Hydration: water is the solvent
Hydrates: crystalline compounds that have ratios of water molecules
Addition of heat to the crystal evaporates the water and leaves an anhydrous salt
Dissolution in water, water molecules in the crystal leave and join the water as a solute, leaving precipitate at the bottom
The behavior of a solution made from a hydrate and the behavior of a solution made from the anhydrous salt form is the same
They both dissolve and create a system with hydrated ions and water molecules
Ionic Compounds
Typically don’t dissolve in nonpolar solvents since the forces are too weak to attract and separate the ions of the solid
Differences in bonding, polarity, and intermolecular forces prevent dissolution between 2 substances
Ethanol is pretty lit
Water is better at dissolving polar and ionic compounds
Ethanol’s nonpolar region makes it better than water at dissolving less-polar substances
Ethanol will dissolve in water because of ethanol’s polar -OH component. The polarity of this part of the ethanol molecule allows Hydrogen bonds to form between ethanol molecules and water molecules. On the other hand, carbon tetrachloride will not as it lacks polarity.
Factors that Affect the Solubility of Gas Solute in a Liquid Solvent
Henry’s Law: solubility of a gas in liquid is tethered to the partial pressure of the gas on the surface of the liquid
This law is applicable when temperature is CONSTANT and only to Gas-Liquid solutions
If the gas has no reaction, it will dissolve like there aren't any other gases near it.
Pressure
Increase in pressure increases the solubility of gas.
When gas is put under pressure, more of its molecules will dissolve into the liquid. This increase in the amount of solute dissolving will cause the rate of solute escaping to increase as well.
This forces the equilibrium to be at a higher rate of solubility than before.
Temperature
As temperature increases, the solubility of Gas decreases.
Temperature increase causes kinetic energy increase, thus increasing the rate of solvent molecules escaping the surface of the liquid and into the atmosphere.
This creates an equilibrium with less gas molecules dissolved in the liquid.
Ex: If a warm bottle of soda and a cold bottle of soda are opened, the warm bottle will effervesce more because of the increased kinetic energy that comes with the increase in temperature. As time goes on, carbon naturally escapes from the liquid and into the atmosphere in attempts to reach a state of equilibrium. Warming the drink up simply expedites this process.
Effervescence: the rapid escape of gas from a liquid it was dissolved in
Ex: opening a can of soda will reduce the pressure back to 1 atm as opposed to 5-10 atm in the unopened can.
Energy Changes Following Solution Formation
Enthalpy of Solution:the net amount of energy absorbed by a solution when a part of a solute dissolves
Negative enthalpy of solution = energy release, exothermic reaction (NaOH in water becomes hot)
Positive enthalpy of solution = energy absorption, endothermic reaction (KI in water becomes cold)
Solvated Molecules: a solute molecule surrounded by solvent molecules
Ex: When a solute molecule is solvated, energy is being released as the bonds holding the solute molecule together are broken.
CONCENTRATION OF SOLUTIONS
Concentration: The amount of solute in a given amount of solvent
The concentration of solute matters because for example with medications, the wrong concentration of drugs can kill the patient.
“Dilute” or “concentration” are not definite terms to describe solutions
“Dilute” - There is a small amount of solute in a solvent
“Concentrated” - There is a large amount of solute in a solvent
These terms are unrelated to the degree to which a solution is saturated
Molarity (M) is moles of solute per liter of solution
To relate the molarity of the solution to the mass of the solute, you must know the molar mass of the solute
1 molar solution of NaOH means that there is one mole of NaOh in every L of the solution
Ex: 1 mole of NaOh has a mass of 40.0g. If this dissolves in enough water to make exactly 1.00 L of solution, the solution will be a 1 M solution (1 mole/1.00 L)
Ex: Half a mole of NaOh has a mass of 20.0 g. If this dissolves in enough water to make exactly 1.00 L of solution, a 0.500 M NaOH solution will be produced (.500 mol/1.00L)
Formula: M = amount of solute (mol) / volume of solution (L)
Note: 1 M solution is not made by adding 1 mol of solute to 1 L of solvent
In this case, the final total volume might not be 1 L
Instead, 1 mol of solute will first be dissolved in a solvent of less than 1 L
The solution will then be diluted with more solvent to bring the total volume to 1 L
Molality (m) is moles of solute per kilogram of solvent
“One modal” solution means that 1 mol of solute dissolves in 1 kg of solvent
Ex: 1 mol of NaOh has a molar mass of 40.0 g when it dissolves in 1 kg of water, we will have a one modal NaOH solution (1 mol/1 kg)
Note: If the solvent is in grams, remember to convert it to kilograms using the conversion factor: 1 kg/1000g
When studying about properties of solutions such as vapor pressure and temperature changes, we use molalities because it is not affected by temperature
Compounds in Aqueous Solutions
Vocabulary
Dissociation: Separation of ions when an ionic compound dissolves in water.
Ionization: Formation of ions from molecular compounds in solution.
Strong/Weak Electrolyte: Degree to which a compound dissociates in water.
Net Ionic Equation: Equation showing only the species that actually participate in a reaction.
Spectator Ions: Ions that do not participate in the chemical reaction.
Hydronium Ion (H₃O⁺): Common ion formed when acids dissolve in water.
Main Concepts
Dissociation of Ionic Compounds
Ionic solids dissociate into individual ions in water.
Ex:
NaCl(s) → Na⁺(aq) + Cl⁻(aq)
CaCl₂(s) → Ca²⁺(aq) + 2Cl⁻(aq)
Calculating Moles of Dissolved Ions
Use the dissociation equation to find the number of moles of ions.
Example:
Al₂(SO₄)₃(s) → 2Al³⁺(aq) + 3SO₄²⁻(aq)
1 mol Al₂(SO₄)₃ produces 5 moles of ions.
Solubility and Precipitation Reactions
Solubility of Ionic Compounds
Some compounds dissolve in water; others don't.
Examples:
Soluble: NaCl, KNO₃
Insoluble: AgCl, CdS
Fig 1.3: Solubility Guidelines
All sodium, potassium, and ammonium compounds are soluble.
Nitrates, acetates, and chlorates are soluble.
Most chlorides are soluble except Ag⁺, Hg₂²⁺, Pb²⁺.
Most sulfates are soluble except Ba²⁺, Sr²⁺, Pb²⁺, Ca²⁺.
Most carbonates, phosphates, sulfides, and silicates are insoluble (there are exceptions!!).
Sulfides of calcium, barium, and magnesium are insoluble.
Precipitation Reactions
Occur when two soluble salts form a precipitate (insoluble product).
Example: (NH₄)₂S(aq) + Cd(NO₃)₂(aq) → CdS(s) + 2NH₄NO₃(aq)
Net Ionic Equations
Shows only ions involved in the chemical change.
Spectator ions are left out.
Example: Full ionic:
Cd²⁺(aq) + 2NO₃⁻(aq) + 2NH₄⁺(aq) + S²⁻(aq) → CdS(s) + 2NO₃⁻(aq) + 2NH₄⁺(aq)
Net Ionic:
Cd²⁺(aq) + S²⁻(aq) → CdS(s)
Pg.416-419: Sage
A Molecular Compound Ionizes in a Polar Solvent
Some molecular compounds can also form ions in solutions (are usually polar compounds)
Ions are formed from solute molecules by the action of the solvent in a process called ionization
Ionization is different from dissociation
When an ionic compound dissolves, the ions that were present separate from one another
When a molecular compound dissolves and ionizes in a polar solvent, ions are formed where none existed in the undissolved compound
Ions formed by such a molecular solute are hydrate; like in aqueous solutions
Energy is released as heat during hydration of the ions, providing energy needed to break the covalent bonds
The general extent to which a solute ionizes in solutions depends on the strength of the bonds within the molecules of the solute and the strength of attraction between the solute and solvent molecules
If the strength of a bond within the solute molecule is weaker than the attractive forces of the solvent molecules, then the covalent bond of the solute breaks and the molecule is separated into ions
EX: hydrogen chloride is a molecular compound that ionizes in aqueous solution
Contains highly polar bond
Attraction between a polar HCl molecule and the polar water molecules is strong enough to break the HCl bond, forming hydrogen ions and chloride ions.
HCl H+(aq) + Cl-(aq)
The Hydronium Ion
Many molecular compounds contain a hydrogen atom bonded by a polar covalent bond
Some of these compounds ionize in an aqueous solution to release H+
The H+ ion attracts other molecules or ions so strongly that it does not normally exist alone
Ionization of hydrogen chloride in water is described as chemical reaction in which a proton is transferred directly from HCl to a water molecule
Where it becomes covalently bonded to oxygen and forms H3O+
H2O(l)+HCl(g) H3O+(aq)+Cl-(aq)
The H3O+ion is known as the hydronium ion
The reaction of the H+ ion to form the hydronium ion produces much of the energy needed to ionize a molecular solute
An Electrolyte’s strength depends on how many dissolved ions it contains
Substances that yield ions and conduct an electric current in solution are electrolytes
Substances that do not yield ions and do not conduct an electric current in solution are non-electrolytes
Hydrogen Chloride is one of a series of compounds composed of hydrogen and the members of Group 17 (halogens)
Hydrogen halides are all molecular compounds with single polar-covalent bonds
All are gases, soluble in water, and electrolytes
Hydrogen chloride, hydrogen bromide, and hydrogen iodide strongly conduct an electric current in an aqueous solution
Hydrogen fluoride only weakly conducts an electric current at the same concentration
Strong Electrolytes
Hydrogen chloride, hydrogen bromide, and hydrogen iodide are 100% ionized in dilute aqueous solution
A strong electrolyte is any compound whose dilute aqueous solutions conduct electricity well; this is due to the presence of all or almost of the dissolved compound in the form of ions.
Are all acids in aqueous solution
Acids, several other acids, and all soluble ionic compounds are strong electrolytes
Distinguishing feature of strong electrolytes is to whatever extent they dissolve in water; yield only ions.
Example some strong electrolytes, such as NaCl, may be highly soluble in water and form ions in solution
Weak Electrolytes
Some molecular compounds form aqueous solutions that contain dissolved ions and some dissolved molecules that are not ionized
Hydrogen fluoride, HF, dissolves in water to give an acidic solution known as hydrofluoric acid
hydrogen -fluorine bond is much stronger than the bonds between hydrogen and other halogens
When hydrogen fluoride dissolves, some molecules ionize
Reverse reaction the transfer of H+ions back to F-ions to form hydrogen fluoride molecules also takes place
HF(aq) + H2O(l) H3O+(aq) + F-(aq)
Concentration of dissolved intact HF is much greater than the concentration of H3O+ and F- ions
Hydrogen fluoride is an example of a weak electrolyte
A weak electrolyte is any compound whose dilute aqueous solutions conduct electricity poorly; this is due to the presence of a small amount of the dissolved compound in the form of ions.
In contrast to a nonelectrolyte, such as the molecular compound sucrose, which dissolves but does not produce any ions in solution
Another example of weak electrolyte is acetic acid
Only a small percentage of acetic acid molecules ionize in aqueous solution
Description of an electrolyte as strong or weak must not be confused with concentrated or dilute
Strong and weak electrolytes differ in the degree of ionization or dissociation
Concentrated and dilute solutions differ in the amount of solute dissolved in a given quantity of a solvent
Colligative Properties of Solutions
Pg.422-427: Pham
Colligative properties: Depend on the concentration of solute particles, not the solute’s identity
The concentration of solute is given in molality
Lower vapor pressure depends on non-electrolyte solute concentration
The boiling and freezing points of a solution are different from a pure solvent
Vapor pressure of the solution is lower than that of the pure solvent at the temperature at which the boiling point occurs for the pure solvent
Solute reduces the freezing point and elevates boiling point
Nonvolatile substance: less tendency to become a gas
According to equilibrium vapor pressure, at the same temperature, the vapor pressure of a solution containing a nonvolatile solute is lower than that of the pure solvent
This is because as we have more solute, the amount of solvent (water) decreases. Fewer water molecules will be available to leave the liquid phase, therefore, the tendency to enter the vapor phase decreases compared to pure water
Equally dilute molal solutions of any nonelectrolyte solutions in the same solvent lower the vapor pressure equally
Ex: Nonelectrolyte glucose lowers the vapor pressure of water by 5.5 x 10-4 atm at 25 degrees C in a 1 molal solution
This shows how the concentration of non-electrolyte solute particles affects vapor pressure, not its identity
As the vapor pressure is lowered, the solution will remain as a liquid over a larger range of temperatures by lowering freezing point and raising boiling point
Vapor pressure, boiling point, and freezing point are all colligative properties
A solution with a nonelectrolyte solute will have a lower freezing point that the pure solvent
Freezing point of a solution with any nonelectrolyte solute is 1.86 degrees C lower than that of water
Ex: When 1 mol of nonelectrolyte dissolves in 1 kg of water, the freezing point decreases from 0.00 to -1.86 degrees C
-1.86 degrees C/m is the molal freezing point constant (K), which is the freezing point depression of the solvent in a 1 molal solution of a nonvolatile, non-electrolyte solute
Each solvent has its own characteristic molal freezing point constant that is the most accurate at 1 atm
Freezing-point depression 𝚫tf: Difference between the freezing points of pure solvent and a solution of a nonelectrolyte in that solvent, directly proportional to the molal concentration
Example: Molal concentration doubles = freezing point depression doubles
Freezing point depression formula: 𝚫tf = Kfm
Kf is expressed in degrees C/m
𝚫tf is expressed in degrees C
A solution with a nonelectrolyte solute will have a higher boiling point than the pure solvent
If the vapor pressure decreases, the boiling point increases
More energy will be required to raise the vapor pressure of a solution to equal the atmospheric pressure
Thus, the boiling point of a solution will be higher than the boiling point of pure solvent
Molal boiling point constant (KB): the boiling point elevation of the solvent in a 1 molal solution of a nonvolatile, nonelectrolyte solute
Molal boiling point constant for water is 0.51 degree C/m
Molal boiling point constants are different for certain solvents
These values are most accurate for dilute solutions
Boiling point elevation 𝚫tb: difference between boiling points of pure solvent and a nonelectrolyte solution of that solvent; directly proportional to the molal concentration
𝚫tb = Kbm
Pg.428-432 vonese
Osmotic pressure is determined by the concentration of dissolved solute particles.
Semipermeable membrane (colligative property): allows the passage of some particles while blocking the passage of others.
By not allowing sucrose molecules to pass through the semipermeable membrane, the rate at which the water molecules move through the membrane and into the solution with sucrose (leaving the pure side) increases
This causes the level of the solution to rise
The level keeps rising until the pressure from the height pushes the water back
The solution stops rising when there an equal rate of water passing through the membrane (equilibrium)
Osmosis: the movement of solvent through a semipermeable membrane from the side of lower solute concentration to the side of higher solute concentration
Occurs when two solutions of different concentrations are separated by semipermeable membrane
Osmotic pressure: external pressure that muse be applied to stop osmosis
Dependent on the concentration of the solute particles, not the type of solute particles which makes it a colligative property
Greater the concentration of solution = greater osmotic pressure of solution
Regulation of osmosis is vital to the life of a cell because cell membranes are semipermeable
Cells lose water and shrink when placed in solution of higher concentration
Swell when placed in a solution of lower concentration
In vertebrates (animals with a backbone), cells are protected from swelling and shrinking by blood and lymph that surround the cells
Blood and lymph are equal in concentration to the concentration inside cell
The total molality of all dissolved particles determines changes in colligative properties.
Some substances affect freezing/boiling points more than expected
Example (with water and freezing points):
0.1 m NaCl lowers FP 2x more than 0.1 m sucrose
0.1 m CaCl2 lowers 3x more
Sucrose = nonelectrolyte -> 1 mol = DF of 1 (1 mol gives 1 mol of particle)
NaCl = strong electrolyte -> 1 mol = DF of 2 (Na+ and Cl-)
CaCl2 -> 1 mol = DF of 3 (Ca2+ and 2Cl-)
Electrolytes produce more particles, causing greater changes in FP/BP
Actual Values for Electrolyte Solutions (ngl this section’s notes sucks but i had trouble trying to make it comprehensible)
Values calculated = expected values
Example:
NaCl (2 ions per unit) lowers FP nearly 2x as much as sucrose (nonelectrolyte)
Compounds that produce 3 ions per unit lower FP nearly 3x as much as sucrose
Strong electrolytes behave almost exactly as expected, based on the number of ions they form in a solution
Attractive forces between dissociated ions affect their movement in solution
The forces are weaker in solution than in solid form but still interfere with ion movement
In very dilute solutions, ions move almost freely due to the large distance between them and weaker attraction
(Peter)Debye-(Erich)Hückel Theory (1923)
Ionic atmosphere: Each ion is surrounded by more ions of opposite charge than like charge
Ions form clusters and behave like a single unit, reducing the effective concentration of ions
High-charge ions attract other ions strongly, so they form stronger clusters and have lower effective concentrations than low-charge ions
441-445: Pham
Properties of Acids and Bases
Acids are identified by their properties
Acids were first recognized as a distinct class of compounds
Sour taste
Taste, however, should never be used to evaluate chemicals
Acids, especially in concentrated solutions, are corrosive (destroy body tissues and clothing)
Acids change color of acid-base indicators
pH will turn colors in acidic solution when used as an indicator (Ex: Turning red when dipped into vinegar, which shows that vinegar is an acid)
React with active metals and release hydrogen gas
Metals above hydrogen in an activity series undergoes single displacement reactions with acids; hydrogen is then formed as a product
Ex: Ba(s) + H2SO4(aq) → BaSO4(s) + H2(g)
React with bases to form salts and water
When chemically equivalent amounts of acids and bases react, three of the properties above disappear because the acid is now neutralized.
Conduct electric current
Acids separate completely into ions in water are strong electrolytes
Others are weak electrolytes
Acid Nomenclature
Binary acid: Contains only two elements: hydrogen and one of the more
Many inorganic acids are binary acids
All hydrogen halides are binary acids
In pure form, these are gases
Binary Acid Nomenclature
Starts with prefix hydro-
The root of the name of the second element follows this prefix
Ends with suffix -ic
Oxyacid: An acid that is a compound of hydrogen, oxygen, and a third element, usually a nonmetal
Although it might seems like the formula shows hydrogen and a polyatomic ion, the hydrogen is actually bonded to oxygen in an oxyacid
Oxyacids are one class of ternary acids
Names of anions are based on names of acids
Some acids are useful in industry
Sulfuric Acid
Most commonly produced industrial chemical
Used in petroleum refining, metallurgy, and manufacture of fertilizer
Essential in production of metals, paper, paint, dyes, detergents, and many chemical raw materials
Used in automobile batteries
An effective dehydrating agent because it attracts water
Sugar and other organic compounds are dehydrated by sulfuric acid
Can cause serious skin burns
Nitric Acid
Pure nitric acid is a volatile, unstable liquid
Dissolving in water makes the acid more stable
Stains proteins yellow (Ex: staining a feather yellow)
Used in making explosives, which are nitrogen containing compounds
Also used in making rubber, plastics, dyes, and pharmaceuticals
Initially it is colorless but will eventually turn yellow because of decomposition to brown NO2 gas
Phosphoric acid
Phosphorus is an essential element for plants and animals
Used for manufacturing fertilizers and animal feed
Sour taste, not toxic
Used as flavoring and cleaning agents
Important in manufacturing detergents and ceramics
Hydrochloric acid
Stomach products HCl
Important for pickling iron and steel; pickling is the immersion of metals in acids to remove stains
Used as cleaning agent in industry, in food processing, in activation of oil wells, in recovering of Mg from seawater, and in production of other chemicals
Concentrated HCl (muriatic acid) can be found in hardware stores
Maintains acidity in pools and cleans masonry
Acetic acid
Pure acetic acid (glacial acetic acid) is a clear, colorless, and pungent-smelling liquid and has a freezing point at 17 degrees Celsius
Forms crystals in cold room
Fermentation of plants produces vinegar that contain acetic acid
White vinegar contains 4-8% acetic acid
Synthesizes chemicals used in manufacture of plastics
Raw material in production of food supplements; ex: lysine
Also used as a fungicide
Properties of bases differ from acids
Bitter Taste
Causatic, meaning they attack skin and tissues to cause severe burns
Change acid-base indicators blue
Sodium Hydroxide (strong base) turns pH paper blue
Dilute aqueous solutions of bases feel slippery
Soap feels slippery when in contact with water
Bases react with acids to produce water and salts
Properties of the original base disappear after the addition of equal part acid
Bases conduct electricity
Bases also form ions in aqueous solutions just like acids. Both acids and bases are electrolytes.
Arrhenius acids and bases produce ions in solution
Aqueous solutions of acids and bases conduct electric current (making them electrolytes
Arrhenius Acid: chemical compound that increases the concentration of hydrogen ions, H+ in aqueous solution
Arrhenius Base: increases the concentration of hydroxide ions, OH- in aqueous solution.
Some bases are Ionic Hydroxides that dissociate in solution to release hydroxide ions.
Other bases bond with a hydrogen ion from water, leaving hydroxide ions floating in the solution.
Aqueous Solutions of Acids
Arrhenius definition: molecular compounds with ionizable hydrogen atoms.
Water solutions of arrhenius acids are known as aqueous acids and ALL of them are electrolytes.
H2O molecules attract 1 or more H+ from acid molecules because acid molecules are sufficiently polar, leaving behind negatively charged anions in the solution.
Strength of Acids
Acid strength is not dependant on how many total hydrogen atoms are in the formula, but how many of those actually ionize completely
Example: phosphoric acid has 3 hydrogens/1 molecule, but NONE of them ionize completely in solution, making it a weak acid.
Strong Acids ionize completely in aqueous solution
Strong Acid=Strong Electrolyte
Increased acid strength=increased polarity=decreased bond energy
Examples of strong acids: perchloric acid (HClO4). Hydrochloric acid (HCl) and nitric acid (HNO3)
Weak acids ionize partially in aqueous solution & only release few hydrogen ions
Aqueous solution of a weak acid contains hydronium ions, anions, and dissolved acid molecules
Weak Acid = Weak Electrolyte
Decreased acid strength=decreased polarity=increase bond energy
Examples of Weak acids: hydrocyanide HCN
Ionization reaction and reverse reactions occur simultaneously with weak acids
Organize acids
Include the carboxyl group
Generally weak acids
Ex: CH3COOH ionizes slightly in water to give hydronium ions and CH3COO-
Even though the chemical formula of CH3COOH + water has 4 hydrogens present, only 1 of those four ionizes, making it a weak acid.
Define acidic hydrogen: the 1 hydrogen/ 4 in CH3COOH that can ionize in H2) to create hydronium
Aqueous Solutions of Bases
Most bases are ionic compounds that contain metal cations and the hydroxide anion, OH-
Most bases are ionic and dissociate when dissolved in water
Strong Basic Solution: a base completely dissociates to yield aqueous OH-ions.
NaOH is a common laboratory base because of its water-solubility and complete dissociation
Note that not all bases are ionic compounds
Something like ammonia, NH3, that’s used as a household cleaner is molecular and still a base since it produces OH- ions when reacting with water molecules
Rules based on period:
All group 1 elements are able to form basic (alkaline) solutions (solutions with a pH over 7)
Nearly all elements in period D are nearly insoluble in water and don’t produce a large amount of hydroxide ions when added in water, preventing them from producing strongly alkaline (basic) solutions
Alkalinity of Solutions
Based on how many hydroxide ions are dissolved in the solution, not the total amount of hydroxide ions in the formula
Strength of Bases
Like Acids, Base strength depends on how much the base dissociates or adds OH- to solution
Strong Bases are also Strong electrolytes & dissociate fully into OH- and another element in dilute aqueous solutions
Typically ionic
Weak Bases are weak electrolytes and don’t dissociate fully in water
Typically molecular
Note that many organic compounds that contain Nitrogen, like NH3 are weak bases (like codeine.)
Base/Acid strength table examples
Name | Solubility in H2O | Electrolyte Strength | OH- concentration | Base/Acid Strength in water |
Ammonia - NH4 | High | weak | low | Weak base |
Cu(OH)2 | Low | strong | low | Weak base |
SAGE 452-456:
Acid-Base Theories
Bronsted-Lowry acids and bases donate or accept protons
Bronsted-Lowry acid- is a molecule or ion that is a proton donor
Because H+ is a proton, all acids as defined by Arrhenius donate protons to water and are Bronsted-Lowry acids as well
Substances other than molecules, such as certain ions, can also donate protons
Such substances are not arrhenius acids but are included in the category of Bronsted-Lowry acids.
Hydrogen chloride acts as a Bronsted-Lowry acid when it reacts with ammonia
The HCl transfers protons to NH3 —-> NH4+ + Cl-
A proton is transferred from the hydrogen chloride molecule, HCl to the ammonia molecule, the ammonium ion, NH4+ is formed.
Electron-dot formulas show the similarity of this reaction to the reaction of HCl with water
In both reactions, hydrogen chloride is a Bronsted-Lowry acid.
Water can also act as a Bronsted-Lowry acid
Example is the following reaction, in which the water molecule donates a proton to the ammonia molecule
Bronsted-Lowry base- is a molecule or ion that is a proton acceptor
In the reaction between hydrocholoric acid and ammonia, ammonia accepts a proton from the hydrochloric acid. It is a Bronsted-Lowry base
Arrhenius hydroxide bases, such as NaOH, are not, strictly speaking, Bronsted-Lowry bases
instead , it is the OH- ion produced in solution that is the Bronsted-Lowry base
It is the species that can accept a proton
Bronsted-Lowry acid-base reaction- protons are transferred from one reactant (the acid) to another (the base)
The figure above shows the reaction between the Bronsted-Lowry acid HCl and the Bronsted-Lowry base NH3
Monoprotic and Polyprotic Acids
Monoprotic acid- an acid that can donate only one proton (hydrogen ion) per molecule
Perchloric acid HClO4, hydrochloric acid, HCl, and nitric acid, HNO3, are all monoprotic
The following equations shows how a molecule of the monoprotic acid HCl donates a proton to a water molecule.
HCl ionizes to form H3O+ ions and Cl- ions. The Cl- has no hydrogens to lose, so HCl has only one ionization step
HCl (g) + H2O (l) —-----> H3O+ (aq) + Cl-(aq)
Polyprotic acid- is an acid that can donate more than one proton per molecule
Sulfuric acid, H2SO4, and phosphoric acid, H3PO4, are examples of polyprotic acids
The ionization of a polyprotic acid occurs in stages
Acid loses its hydrogen ions one at a time
Sulfuric acid ionizes in two sages
In its first ionization, sulfuric acid is a strong acid
It is completely converted to hydrogen sulfate ions, HSO4-
H2SO4(l)+H2O(l) H3O+(aq)+HSO4-(aq)
The hydrogen sulfate ion is itself a weak acid. It establishes the following equilibrium in solution
HSO4-(aq)+ H2O(l) H3O+(aq)+SO42-(aq)
All stages of ionization of a polyprotic acid occur in the same solution
Sulfuric acid solutions therefore contain H3O+, HSO4-, and SO42- ions.
Note that in sulfuric acid solutions, there are many more hydrogen sulfate and hydronium ions than there are sulfate ions.
Diprotic acid-sulfuric acid is the type of polyprotic acid that can donate two protons per molecule, and it is therefore known as a diprotic acid
Tripotic acid-phosphoric acid is the type of polyprotic acid, an acid able to donate three protons per molecule
The equations for these reactions are shown below.
A solution of phosphoric acid contains H3O+, H3PO4, H2PO4-, HPO42-, and PO43-
As with most polyprotic acids, the concentration of ions formed in the first ionization is the greatest
There are lesser concentrations of the respective ions from each succeeding ionization.
A Lewis acid or base accepts or donates a pair of electrons
The arhenius and Bronsted-Lowry defintions describe most acids and bases
Both definitions assume that the acid contains or produces hydrogen ions
A third acid classification, based on bonding and structure, includes, as acids, substances that do not contain hydrogen at all
Lewis acid- is an atom, ion, or molecule that accepts an electron pair to form a covalent bond
Applies to any species that can accept an electron pair to form a covalent bond with another species
The formula for a lewis acid need not include hydrogen. Even a silver ion can be a Lewis acid, accepting electron pairs from ammonia to form covalent bonds
Any compound in which the central atom has three valence electrons and forms three covalent bonds can react as a lewis acid
It does so by accepting a pair of electrons to form a fourth covalent bond, completing an electron octet
ACID-BASE DEFINITIONS
Type | Acid | Base |
Arrhenius | H+ or H3O+ producer | OH- producer |
Bronsted-Lowry | Proton (H+) donor | Proton (H+) acceptor |
Lewis | Electron-pair acceptor | Electron-pair donor |
Lewis Base- is an atom, ion, or molecule that donates an electron pair to form a covalent bond
An anion is a lewis base in a reaction in which it forms a covalent bond by donating an electron pair
Lewis acid-base reaction- is the formation of one or more covalent bonds between an electron-pair donor and an electron-pair acceptor
Although the three acid-base definitions differ, many compounds may be categorized as acids or bases according to all three descriptions
457-463: vovo
Brønsted-Lowry reactions involve conjugate acid-base pairs.
The Brønsted-Lowry theory is useful for describing acid-base reactions in aqueous solutions
When a Brønsted-Lowry acid gives up a proton, the remaining ion or molecule that re-accepts the proton is the conjugate base.
Thus, the species that remains after a Brønsted-Lowry acid has given up a proton is the conjugate base of that acid.
The species that is formed when a Brønsted-Lowry base gains a proton is the conjugate acid of that base.
Brønsted-Lowry acid-base reactions are equilibrium systems (work both ways) involving two conjugate acid-base pairs.
In every conjugate acid-base pair, the acid has one more proton than its conjugate base.
Strength of Conjugate Acids and Bases
The extent of the Brønsted-Lowry acid-base reaction depends on the relative strengths of the acids and bases involved.
Strong acids readily donate protons, resulting in very weak conjugate bases.
Strong bases have little tendency to attack and retain protons, resulting in very weak conjugate acids.
General rule: the stronger an acid is, the weaker the conjugate base, and the stronger the base, the weaker the conjugate acid. (Strong-weak, weak-strong)
Using Strength to Predict Reactions
The relative strengths of acids and bases can be used to predict the outcome of a reaction.
The stronger acid reacts with the stronger base to produce the weaker acid and the weaker base.
Proton-transfer reactions favor the production of the weaker acid and the weaker base.
For an acid-base reaction to form products completely, the reactants must be much stronger acids and bases than the products.
Some substances act as either acids or bases.
Amphoteric: any species that can react as either an acid or a base.
Water is a common example.
Whether a substance acts as an acid or a base depends on the strength of the acid or base it is reacting with.
Strong acid - act as a base
Strong base - act as an acid
-OH in a Molecule
Molecular compounds containing OH groups can be acidic or amphoteric
Hydroxyl group: covalently bonded -OH group in an acid
For a compound to be acidic, a water molecule must be able to attract a hydrogen atom from a hydroxyl group.
More likely when O-H is very polar
Any feature of a molecule that increases the polarity of the O-H bond increases the acidity of the compound.
Small, more electronegative nonmetal atoms at the upper right of the periodic table form compounds with acidic hydroxyl groups.
Oxyacids are molecular electrolytes that contain onerous or more O-H bonds, such as chloric and perchloric acids.
The number of oxygen atoms bonded to the atom connected to the -OH group affects the compound’s behavior.
More oxygen - increase acidity
Electronegative oxygen atoms draw electron density away from the O-H bond, making it more polar.
Example:
In acetic acid, a second oxygen atom is bonded to the carbon atom connected to the -OH group, making it acidic, unlike ethanol.
Neutralization reactions produce water and a salt.
Neutralization reactions involve the reaction of an acidic compound with a basic compound (they neutralize each other)
Example:
NaHCO3 and H2C4H4O6 produce baking powder
When water is added, produces carbon dioxide
Strong Acid-Strong Base Neutralization
Acid-base reactions occur in aqueous solutions between strong acids and strong bases.
Example (ngl i was confused by the long example):
In this reaction, there was an equal number of H3O+ and OH- ions and they are fully converted to water.
Both a salt and water were produced.
In aqueous solutions, neutralization is the reaction of hydronium ions and hydroxide ions to form water molecules.
Salt: An ionic compound composed of a cation from a base and an anion from an acid.
Acid Rain
Industrial processes produce gases like NO, NO2, CO2, SO2, and SO3.
These gases dissolve in atmospheric water to produce acidic solutions, resulting in acid rain or snow.
Rainwater is normally slightly acidic (very acidic rain is acid rain).
Acid rain erodes statues and affects ecosystems, such asthe water environment and forests
Acid rain can decrease the biodiversity of exosystems by eliminating fish populations in lakes and streams.
Amendments to the Clean Air Act in 1990 set limits on SO2 emissions from power plants, which have decreased but not eliminated acid rain in the US.
Aqueous Solutions and the Concept of pH
471-477: Sage
Self-ionization of water forms hydronium and hydroxide ions
Hydronium and hydroxide ions are also provided by the solvent, water
Self-ionization of water
Pure water is an extremely weak electrolyte
Water undergoes self-ionization
Self ionization of water- two water molecules produce a hydronium ion and a hydroxide ion by a transfer of a proton
The following equilibrium takes place
H2O(l) + H2O(l) H3O+ (aq) + OH-(aq)
Conductivity measurements show that concentrations of H3O+ and OH- in pure water are each only 1.0 x 10⁻⁷ mol/L of water at 25⁰C.
The formula of the particular ion or molecule is enclosed in brackets, [ ].
The mathematical product of [H3O⁺] and [OH⁻] remains constant in water and dilute aqueous solutions at constant temperature
This constant mathematical product is called the ionization constant of water, Kw and is expressed by the following equation
Kw = [H3O⁺][OH⁻]
For example, in water and dilute aqueous solutions at 25⁰C, the following relationship is valid
Kw = [H3O⁺][OH⁻] = (1.0 x 10⁻⁷)(1.0 x 10⁻⁷) = 1.0 x 10⁻¹⁴
The ionization of water increases as temperature increases. Therefore the ion product Kw also increases as temperature increases
However at any given temperature Kw is always a constant value.
The value 1.0 x 10⁻¹⁴ is assumed to be constant within the ordinary range of room temperatures
Neutral, Acidic, and Basic Solutions
Because the hydronium ion and hydroxide ion concentrations are the same in pure water, it is neutral
Any solution in which [H3O⁺]= [OH⁻] is neutral
Solutions in which the [H3O⁺] is greater than the [OH⁻] are acidic
Bases increase the concentration of OH⁻ in aqueous solutions
In basic solutions, the [OH⁻] is greater than the [H3O⁺].
Calculating [H3O⁺] and [OH⁻]
Strong acids and bases are considered completely ionized or dissociated in weak aqueous solutions
NaOH(s) (H2O) Na⁺(aq) + OH⁻(aq)
Because NaOH is a strong base, 1 mol of it will yield 1 mol of OH⁻ in an aqueous solution.
Therefore a 1.0 x 10⁻² M NaOH solution has an [OH⁻] of 1.0 x 10⁻²M, as shown by the following
1.0 x 10-2mol NaOH1 L solution x 1 mol OH⁻1 mol NaOH = 1.0 x 10-2 mol OH⁻1 L solution = 1.0 x 10⁻²M OH⁻
Notice that the [OH⁻] is greater than 1.0 x 10⁻⁷ M. This solution is basic
Because the Kw of an aqueous solution is a relatively constant 1.0 x 10⁻¹⁴ at ordinary room temperatures, the concentration of either ion can be determined if the concentration of the other ion is known
EX
Kw = [H3O⁺][OH⁻] = 1.0 x 10⁻¹⁴
[H3O⁺] = 1.0 x 10⁻¹⁴[OH⁻]= 1.0 x 10⁻¹⁴1.0 x 10⁻²=1.0 x 10⁻¹²M
The [OH-], is 1.0 x 10-2 M, is greater than the [H3O+], 1.0 x 10-12 M, as is true for all basic solutions
IN SHORT the [H3O+] is greater than [OH-] for all acidic solutions and vise versa for {OH-]
In order for Kw to remain constant, an increase in either the [H3O+] or the [OH-] in an aqueous solution causes a decrease in the concentration of the other ion.
The concentrations of hydronium and hydroxide ions determine pH and pOH
pH also indicates the hydronium ion concentration of a solution
The pH of a solution is defined as the negative of the common logarithim of the hydronium ion connetration, [H3O+]
pH= -log[H3O+]
The common logarithim of a number is the power to which 10 must be raised to equal the number
A neutral solution at 25oC has a [H3O+] of 1 x 10-7 is -7.0. The pH is determined as follows
pH= -log [H3O+] = -log (1.0 x 10-7) = -(-7.0) = 7.0
pOH of a solution is defined as the negative of the common logarithim of the hydroxide ion concentration, [OH-]
pOH=-log[OH-]
EX: suppose the [H3O+] in a solution is greater than the [OH-], as is true for acidic solutions.
For example, the pH of an acidic solution at 25oC with a [H3O+] of 1 x 10-6 M is 6.0.
pH= -log[H3O+] = -log(1 x 10-6) = - (-6.0 = 6.0
Solution | General Condition | At 250C |
Neutral | [H3O+] = [OH-] pH=pOH | [H3O+] = [OH-] = 1 x 10-7 M pH = pOH = 7.0 |
Acidic | [H3O+] > [OH-] pH < pOH | [H3O+] > 1 x 10-7 M [OH-] < 1 x 10=7 M pH < 7.0 pOH > 7.0 |
Basic | [H3O+] < [OH-] pH > pOH | [H3O+] < 1 x 10-7 M [OH-] > 1 x 10-7 M pH > 7.0 pOH < 7.0 |
As the temperature changes, the exact values will change because the value of Kw changes. However, the relationship pH + pOH = pKw will remain the same.
477-481: Em
The sum of a solution’s pH and pOH is always 14
If you’re given the [H3O+] you can always find the pH and vice versa
pH represents a logarithm
Because of this, the number to the LEFT of the decimal of the pH determines where the decimal point is in the [H3O+]
The amount of sig figs of the [H3O+] value determines how many figures there are to the RIGHT of the decimal point in the pH
Ex: [H3O+]=1 X 10^-7
this value in scientific notation has 1 SIG FIG. Thus, the -log of [H3O+], aka the pH will have 1 DIGIT to the right of the decimal
pH=7.0
Example 1: Calculating the pH of a solution given the Identity and concentration of the solution
Calculating pH from [H3O+] when [H3O+] is an integral power of ten
In the simplest types of solution, the value of [H3O+] is an integral power of 10 like 1M or .01 M
The pH of this type of solution is just the exponent of the [H3O+] with the sign changed
Ex: [H3O+] = 1X10^-5 → pH=5.0
Calculating the pH from [H3O+] when [H3O+] is NOT an integral power of ten
Must use your calculator!!
Sig Fig rules:
# of zeros between the decimal point and first sig fig of [H3O+]’s general form is the number to the LEFT of the decimal point in your pH
# of sig figs of [H3O+] tell you how many places to the RIGHT of the decimal point
You can always check the accuracy of your pH calculation by estimating the pH using [H3O+] values that have integral numbers as their pH
Ex: Given [H3O+] = 3.4 X 10^-5
3.4 X 10^-5 is between 1 X 10^-4 and 1 X 10^-5
1 X 10^-4 = pH of 4.0 and 1 X 10^-5 = pH of 5.0
Thus, the pH of the given solution must be between 4.0 and 5.0
Example 2: Calculating the pH of a solution given the [H3O+] of the solution
Calculating [H3O+] and [OH-] from pH
Basic formula: pH=-log[H3O+]
Since the base of common logs is 10, the antilog of a common log is 10 raised to that number
ex: log[H+]=-pH → [H+]=antilog(-pH) → [H+]=10^(-pH)
When the pH is an integer, the exponent of 10 that gives [H+] is the negative of the pH
ex: When [H+]=1x10^-7 M, pH=7.0
ex: When [H+]=1x10^0 M aka 1 M , pH=0
Example 3: calculation [H3O+] given pH that’s an integral number
Example 4: Calculating Hydronium concentration, hydroxide concentration, and acidity given the pH of the solution that’s NOT an integral number
pH Calculations in accordance with Acid and Base Strength
Because KOH is a soluble ionic compound and a strong base:
KOH’s molarity directly tells you the [OH-]
note that knowing the [OH-] allows you to determine the [H+] and therefore the pH
The experimental pH of this solution = calculated (theoretical) pH of this solution
Note that these properties apply to STRONG ACIDS as well, such as HCl hydrochloric acid
Because CH3COOH’s molecules don’t fully ionize and it’s a weak acid:
CH3COOH’s molarity (M) does NOT directly tell you the [H+]
Thus, the calculated (theoretical) pH of this solution =/= experimental pH of this solution
To find the [H+] and [OH-], you must find the pH of the solution experimentally
These properties apply to WEAK BASES as well, such as NH3 ammonia
Lab note: Drano is an arrhenius base483-487: pham
DETERMINING PH AND TITRATIONS
Indicators can determine pH, pOH, and strength
Approximate value for pH can be determined using acid-base indicators
Acid-base indicators: compounds whose colors are sensitive to pH
Indicators change colors because they are either weak acids or weak bases
A weak acid indicator (HIn) can be represented:
HIn ⇌ H+ + In-
In- is the anion of the indicator
Colors result from the fact that HIn and In- are different colors
In- ions are Bronsted bases
Indicator is present in largely nonionized form, HIn
In basic solutions, OH- ions combine with H+ ions produced by indicator; this makes the indicator molecules to further ionize to makeup the loss of H_ ions
Indicator is now largely present in In- (blue)
Transition interval: pH range in which an indicator changes color
Different pH indicators change color at different pH values
Color depends on the amounts of HIn and In- at a given pH
Ex: Methyl red changes color between pH 4.4 and 6.2
At pH 4.4 or below, the indicator mostly exists as HIn since it is acidic (red)
Above pH 6.2, it mostly exists as In- since it is basic (yellow)
During the transition interval, the amounts of both forms are the same so the color is resulted due to the mixture of HIn and In-
A pH meter is a more precise tool to measure pH levels
pH meter: determines pH level by measuring the voltage between two electrodes that were placed in the solution
Voltage changes as H3O+ concentration changes
Titration is used to determine exact concentrations
Neutralization occurs when 1 mol of H3O+ (acid) equally reacts with 1 mol of OH- (base) to create 2 mols of water
As we mix two solutions together, solution of H3O+ with red pH indicator red and solution of OH- with blue pH indicator, this would create a neutral solution with a pH indicator of green
When adding 0.10 mol of NaOH to 1 L of 0.10 M HCl solution, H3O+ and OH- ions will get equally released and combine until the product [H3O+][OH-] returns to the value of 1 x 10-14
NaCl is the product
Titration: controlled addition and measurement of the amount of a solution of known concentration required to react completely with a measured amount of a solution of unknown concentration
Helps determine the chemically equivalent amounts of acid and base
488-493: Vonese
Equivalence Point
Equivalence point: the point at which the two solutions used in a titration are present in chemically equivalent amounts
Indicators and pH meters can be used to determine the equivalence point
pH will change readily as the equivalence point is approached
If an indicator is used, must change color over a range that includes pH of equivalence point
End point of an indicator: the point in a titration at which an indicator changes color
Indicators:
Litmus: changes at pH 7, but broad color-change internal (pH 5.5-8.0)
Bromthymol blue: better, smaller transitional interval (pH 6.2-7.6)
Strong-acid/strong-base titration:
The neutralization produces a salt solution with a pH of 7, so indicators that undergo transition at about pH 7 are used
Strong-acid/weak-base titrations:
The equivalence point is acidic (pH lower than 7) because the salt formed is a weak acid.
Methyl orange is an example of an indicator used for this
Weak-acid/strong-base titrations:
These reactions produce salt solutions with a pH greater than 7 because the salt formed is a weak base
Indicators include Phenolphthalein (turns pink) and Menthyl red (turns red)
Weak-acid/weak-base titrations:
The pH at the equivalence point can be acidic, basic, or neutral, depending on the relative acid-base strengths
Not practical to carry out these transitions because pH value does not change dramatically
No indicator typically used
As base is added, the pH changes from a lower numerical value to a higher one.
The change in pH occurs slowly at first, then rapidly through the equivalence point, and then slowly again as the solution becomes more basic.
A standard solution is used to titrate unknowns.
Standard solution: The solution that contains the precisely known concentration of a solute
To be certain of the concentration of the known solution, a solution must first be compared with a solution of a primary standard
Primary standard: a highly purified solid compound used to check the concentration of the known solution in a titration.
Known solution is prepared first to give approximately desired concentration before it is determined precisely by titrating carefully measured quantity of the primary standard
Thermochemistry
501-508: Sage
Thermochemistry- is the study of the transfers of energy as heat that accompany chemical reactions and physical changes
Temperature and heat are related but not identical
Calorimeter- the energy absorbed or released as heat in a chemical or physical change is measured in a calorimeter
Energy given off (or absorbed) during the reaction is equal to the energy absorbed (or given off) by the known quantity of water.
Amount of energy is determined from the temperature change of the known mass of surrounding water
Data collected from calorimetry experiments are temperature changes because energy cannot be measured directly; but temperature, is directly measurable
Temperature- is a measure of the average kinetic energy of the particles in a sample of matter.
The greater the kinetic energy of the particles in a sample, the higher the temperature is and the hotter it feels
To assign numerical values to temperature, it is necessary to define a temperature scale.
For calculations in thermochemistry, we use the Celsius and Kelvin scales.
Celsius and Kelvin temperatures are related by the following equation
K= 273.15 + oC
The ability to measure temperature is thus based on energy transfer; measured in joules
A joule is the SI unit of heat as well as all other forms of energy
N x m = kg x m2s2
Heat can be thought of as the energy transferred between samples of matter because of a difference in their temperatures
Energy transferred as heat always moves spontaneously from matter at a higher temperature to matter at a lower temperature
Energy transfer varies from reaction to reaction
The quantity of energy transferred as heat during a temperature change depends on the nature of the material changing temperature, the mass of the material changing temperature, and the size of the temperature change
EX: one gram of iron heated to 100.0oC and cooled to 50.0oC in a calorimeter transfers 22.5 J of energy to the surrounding water.
But one gram of silver transfers 11.8 J of energy under the same conditions.
Difference depends on the metals’ differing capacities for absorbing this energy.
Specific heat is the amount of energy required to raise the temperature of one gram of a substance by one Celsius degree (1oC) or one kelvin (1 K) (because the sizes of the degree divisions on both scales are equal)
Values of specific heat can be given in units of joules per gram per Celsius degree, J/(g x oC), joules per gram per kelvin, J/(g x K), or calories per gram per Celsius degree, cal/(g x oC)
Specific heat is measured under constant pressure conditions, so its symbol, cp, has a subscripted p as a reminder. In the equation, cp is the specific heat at a given pressure, q is the energy lost or gained, m is the mass of the sample, and T represents the change in temperature
cp=qm x T
This equation can be rearranged to give an equation that can be used to find the quantity of energy gained or lost with a change in temperature
Energy Lost or Gained q=cp m T
Heat energy is transferred during a reaction
Energy absorbed as heat during a chemical reaction at constant pressure is represented by H. The H is the symbol for quantity called enthalpy. It is not practical to talk about enthalpy as a quantity, because we have no way to directly measure the enthalpy of a system
Only changes in enthalpy can be measured. The Greek letter (“delta”) stands for “change in.”
Therefore, H is read as “change in enthalpy”.
An enthalpy change is the amount of energy absorbed by a system as heat during a process at constant pressure H = Hproducts- Hreactants
The enthalpy of reaction is the quantity of energy transferred as heat during a chemical reaction. (HEAT OF REACTION)
Enthalpy of Reaction in Exothermic Reactions
If a mixture of hydrogen and oxygen is ignited, water will form and energy will be released explosively
Energy that is released comes from the reactants as they form products
Because energy is released, the reaction is exothermic, and the energy of the product, water, must be less than the energy of the reactants.
EX: 2 mol of hydrogen gas at room temp are burned, 1 mol of oxygen gas is consumed and 2 mol of water vapor are formed
2H2(g) + O2(g) —> 2H2O(g)
Experiments have shown that 483.6 kJ of energy are evolved when 2 mol of gaseous water are formed from its elements at 298.15 K.
Modifying the chemical equation to show the amount of energy as heat released during the reaction gives the following expression.
2H2(g) + O2(g) —> 2H2O(g) + 483.6 kJ
This expression is an example of a thermochemical equation, an equation that includes the quantity of energy released or absorbed as heat during the reaction as written
In any thermochemical equation, we must always interpret the coefficients as numbers of moles and never as numbers of molecules.
The quantity of energy released as heat in this or any other reaction depends on the amount of reactants and products
The quantity of energy as heat released during the formation of water from H2 and O2 is proportional to the quantity of water formed; require twice as many moles of reactants and would release 2x more energy as heat
4H2(g) + 2O2(g) —> 4H2O(g) + 967.2 kJ
Producing one-half as much water requires one-half as many moles of reactants and releases only one-half as much energy, or ½ x 483.6 kJ.
H2(g) + ½ O2(g) —> H2O(g) + 241.8 kJ
Enthalpy of Reaction in Endothermic Reactions
The situation is reversed in an endothermic reaction– products have a larger enthalpy than reactants.
Decomposition of water vapor is endothermic; it is the reverse of the reaction that forms water vapor
In endothermic reactions, enthalpy now appears on the reactant side of the thermochemical equation but no changed value
2H2,O(g) + 483.6 kJ —> 2H2(g) + O2(g)
The physical states of reactants and products must always be included in thermochemical equations because they influence the overall amount of energy as heat gained or lost.
EX: energy need to decompose water would be greater than 483.6 kJ if we started with ice, becauze extra energy would be needed to go from ice to liquid and then to vapor
Thermochemical Equations
Thermochemical equations are usually written by designating the value of H
For exothermic reaction, H is always negative because the system loses energy.
2H2(g) + O2(g) —> 2H2O(g) H= -483.6kJ
For endothermic reaction H is always positive because the system gains energy.
2H2O(g) —-> 2H2(g) + O2(g) H= +483.6kJ
Since energy as heat is absorbed, the enthalpy of the reactants is lower than the final enthalpy of the products, and H is positive.
When looking at all the thermochemical equations, consider the following.
The coefficients in a balanced thermochemical equation represent the number of moles of reactants and products and never the number of molecules. They can be fractions when necessary
The Physical state of the product or reactant involved in a reaction is an important factor, and, therefore, must be included in the thermochemical equation
The change in Enthalpy represented by a thermochemical equation is directly proportional to the number of moles of substances undergoing a change. If 2 mol of water are decomposed, twice as much enthalpy
The value of the enthalpy change, H, is usually not significantly influenced by changing temperature
Enthalpy of formation is the energy change when elements form one mole of a compound
Thermochemical data are often recorded as the enthalpies of such composition reactions.
The molar enthalpy of formation is the enthalpy change that occurs when one moles of a compound is formed from its elements in their standard state at 250C and 1 atm.
Enthalpies of formation are given for the standard states of reactants and products; usually atmospheric pressure and room temp 298.15K
To signify that a value represents measurements on substances in their standard states, a o sign is added to the enthalpy symbol, giving delta H for the standard enthalpy of a reaction.
Adding a subscript f, as in Hfo further indicates a standard enthalpy of formation.
508-514 (start from “exothermic compounds tend to be very stable”): Pham
Exothermic compounds tend to be very stable
A compound with a large negative enthalpy of formation releases a large amount of energy as heat when it’s formed → stable
Elements in standard states have ∆H0f = 0
The majority of enthalpies of formation are negative
∆H0f of CO2 is -393.5 kJ/mol therefore, it is more stable than the elements from which it was formed
Compounds with relatively positive or slightly negative values are unstable
Ex: HI is a colorless gas that decomposes at room temperature
Has an enthalpy of formation of +26.5 kJ/mol
As it decomposes, the violet iodine vapor, I2, becomes visible through the container
Compounds with a high positive enthalpy of formation are very unstable and may react or decompose violently
Ex#1: C2H2 reacts violently with oxygen and must be stored in cylinders as a solution in acetone
Ex#2: HgC2N2O2 has a very large enthalpy of formation of +270 kJ/mol which makes it useful as a detonator for explosives
Enthalpy changes in combustion
Combustion reactions produce energy in the form of light and heat when a substance is combined with oxygen
Enthalpy of combustion: the enthalpy change that occurs during the complete combustion of one mole of a substance
Enthalpy of combustion is defined in terms of one mole of reactant, and enthalpy of formation is defined in terms of one mole of product
All substances are in their standard states
General enthalpy notation, ∆H, applies to enthalpies of reaction
∆Hc refers to the enthalpy of combustion
A combustion calorimeter is a common instrument used to determine enthalpies of combustion
A similar apparatus under constant pressure is used to obtain enthalpy measurements
Change in enthalpy is calculated using Hess’s Law
Thermochemical equations can be rearranged and added to give enthalpy changes for reactions not included in the data tables
Hess’s law: the overall enthalpy change in a reaction is equal to the sum of enthalpy changes for the individual steps in the process
Energy difference between reactants and products is independent of the route
Measured enthalpies of reaction can be combined to calculate enthalpies of reaction that are difficult or impossible to actually measure
Calculate the enthalpy of formation of methane gas from its elements, hydrogen gas and solid carbon (graphite), at 298.15 K:
In order to calculate the change in enthalpy, we use the combustion reactions of each element
Principles for combining thermochemical equations:
If a reaction is reversed, the sign of ∆H is also reversed
Multiply the coefficients of the known equations so that, when added together, they give the desired thermochemical equation. Multiply ∆H by the same factor as the corresponding equation.
In this case, since methane is on the right side of the thermochemical equation, we must reverse the combustion equation of methane and change the sign of ∆H. This will turn the reaction to an endothermic one.
Since we now have 2 moles of water as a reactant, we will need 2 moles of water as a product
For the combustion of hydrogen, it only produces one mole of water so we would need to multiply everything by 2
Now add the three equations together
Hess’s law states that the enthalpy difference between reactants and products is independent of the pathway
Any enthalpy of reaction may be calculated using enthalpies of formation for all the substances in the reaction of interest, without knowing anything else about how the reaction occurs
Enthalpy of formation is the sum of its sub-reaction enthalpies
When carbon is burned in a limited supply of oxygen, CO is produced
Carbon is first oxidized to CO2 then part of it is reduced with carbon to give some CO
These two reactions occur simultaneously so we get a mixture of CO and CO2
It’s not possible to directly measure the enthalpy of formation of CO(g_ from C(s) and O2(g)
However, we do know the enthalpy of formation of CO2 and enthalpy of combustion of CO
Reverse second equation because we need CO as a product
This diagram is a model for a reaction that takes place in two steps
If we plot the reactions based on their relative energy, you can see the relationship among the values for the enthalpy of formation of CCO
The formation of CO2 is at a level of -393.5 kJ/mol
It shows the reverse of the combustion reaction (+293.0 kJ/mol) is added to that level
The value of the formation of CO is -110.5 kJ/mol.
516-518: Vonese
Driving Force of Reactions
The change in energy of a reaction system is one of the two factors that allow chemists to predict whether a reaction will occur spontaneously and to explain how it occurs
The randomness of the particles in a system is the second factor affecting whether a reaction will occur spontaneously
Reactions generally move to a lower-energy state.
The majority of chemical reactions in nature are exothermic, resulting in products that are more stable and have lower energy than the reactants.
Reactions naturally tend towards a lower energy state
Some endothermic reactions can occur spontaneously, indicating that energy (such as continued heating) is not the only factor determining spontaneity
Entropy measures randomness in a system.
A naturally occurring endothermic process is melting.
Such as an ice cube melting spontaneously at room temperature as energy is transferred from the warm air to the ice
During melting, the well-ordered arrangement of water molecules in the ice crystal is lost, and a less-ordered liquid phase of higher energy content is formed
A system that can go from one state to another without a decrease in enthalpy does so with an increase in entropy
There is a general tendency in nature to proceed in a direction that increases the randomness of a system
A random system lacks a regular arrangement of its parts. Tendency toward randomness is called entropy.
Entropy, S, can be defined in a simple qualitative way as a measure of the degree of randomness of the particles, such as molecules, in a system.
Entropy in states of matter
Solids: particles in fixed position, vibrating; randomness is LOW, so entropy is LOW
Liquids: particles are moving rapidly and are much farther apart; MORE random, entropy is HIGHER in liquids (compared to solids)
Gases: particles are moving rapidly and are much farther apart; MUCH MORE random, entropy is HIGHER in gases
General rule: Entropy in gases>>liquids>solids
(exception of liquid mercury, which is less than some solids)
The entropy of a pure crystalline solid at absolute zero is zero
As energy is added, randomness of molecular motion increases
Measures of energy absorbed and calculations that are used to determine absolute entropy (standard molar energy) are kJ/(mol • K)
Entropy change, which can also be measured, is defined as the difference between the entropy of the products and the reactants.
An increase in entropy is + ΔS.
A decrease in entropy is - ΔS.
The process of forming a solution almost always involves an increase in entropy because there is an increase in randomness.
This applies to mixing gases, dissolving a liquid in another liquid, and dissolving a solid in a liquid
Example: Sugar in tea
Initial: solid sugar has low entropy as molecules are in one region, separate from water molecules
Then: after dissolving, sugar molecules are thoroughly mixed throughout tea; both sugar and water can be found anywhere, increasing system’s randomness and + ΔS
Free energy changes determine if a reaction is endothermic or exothermic.
Processes in nature are driven towards two directions: towards least enthalpy and greatest entropy
The direction that LOWERS free energy of a system will be the direction natural processes proceed in
Enthalpy and Entropy fight for dominance. The dominant factor determines if forward or reverse reaction is favored
Free energy: the combined enthalpy-entropy function, also known as “Gibbs free energy”
Assess the tendency of enthalpy and entropy to change
Free-energy change: denoted by ΔG; defined as the difference between the change in ΔH and TΔS
Typical Measurements for this equation
TΔS: kj or J
ΔH: kj or J
ΔG: kj or J
ΔS: kj/K
There are 4 possible combinations of terms with this formula
Ex1. Exothermic Reaction

Ex2. Endothermic Reaction
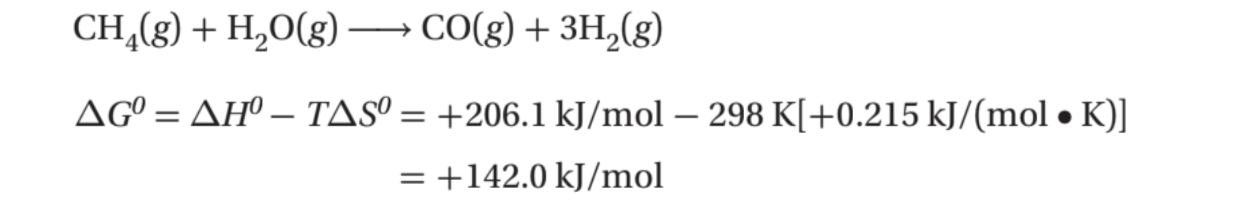
Even though the entropy increase would normally push the forward reaction to occur spontaneously at room temperature, the positive ΔG tells us otherwise
Since ΔH is positive, this rxn is endothermic