UNIT 4: Chemical Bonding and Structure
Unit 4.1 Ionic & Covalent Bonding
4.1.1 Forming Ions
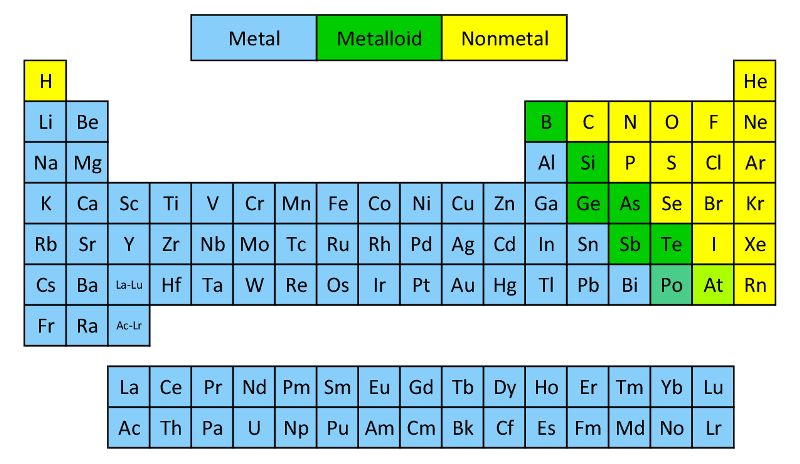
Metals are on the left of the Periodic Table
Lose electrons from their valence shell
Form positively charged cations
Non-metals are on the right of the Periodic Table
Gain electrons
Form negatively charged anions
Ionic Bonds = bonds formed from the transfer of electrons from a metallic element to a non-metallic element
Usually results in both the metal and non-metal having a full outer shell
Thus, these atoms have electronic configurations that are the same as a noble gas (elements with full outer shells)
Example:
Na (Sodium)
It is a metal in group 1, so it has 1 valence electron
After it loses this electron, it becomes Na+ (Sodium Cation)
Cation = A positively charged ion
A sodium ion has the same electronic configuration as neon: [2, 8]
CL (Chlorine)
It is a non-metal in group 17, so it has 7 valence electrons
After it gains an electron from a different atom, it becomes Cl- (Chloride Anion)
Anion = A negatively charged ion
A chloride ion has the same electronic configuration as argon: [2, 8, 8]
Electrostatic Attractions = Attractions formed between the oppositely charged ions (cations and anions) to form ionic compounds
Very strong, so it takes a lot of energy to break
This is why ionic compounds have high melting points
4.1.2 Ionic Compounds
Ionic Lattices
Ionic Lattice = an evenly distributed crystalline structure formed by ions
Ions arranged in a regular repeating pattern due to electrostatic forces of attraction between cations and anions
This causes positive charges to cancel out negative charges, causing the final, overall lattice to be electrically neutral
Properties of Ionic Compounds
Different types of structure/bonding → different physical properties (ie. melting/boiling points, electrical conductivity, and solubility)
Ionic Bonding & Giant Ionic Lattice Structures
Ionic compounds are strong
Their strong electrostatic forces keep ions held together strongly
Ionic compounds are brittle, so ionic crystals can split apart
Ionic compounds have high melting and boiling points
This is because of their strong electrostatic forces between the ions that keep them held strongly together
As charge density of the ions increase, the melting and boiling points increase
This is due to the greater electrostatic attraction of charges
ex. Mg2+O2- has a higher melting point than Na+Cl-
Ionic compounds are soluble in water because they can form ion-dipole bonds
Ionic compounds only conduct electricity when molten or in solution
In those two cases, the ions can freely move around and conduct electricity
However, as a solid, the ions are fixed, so they are unable to move around
4.1.3 Formulae & Names of Ionic Compounds
Review:
Ionic compounds are formed from a metal and a nonmetal bonded together
Ionic compounds are electrically neutral (positive charges = negative charges)
Charges on Positive Ions
Positive Ions:
All metals
Some non-metals
ex. NH4+ (Ammonia) and H+ (Hydrogen)
Charges of ions depend on their position in the Periodic Table:
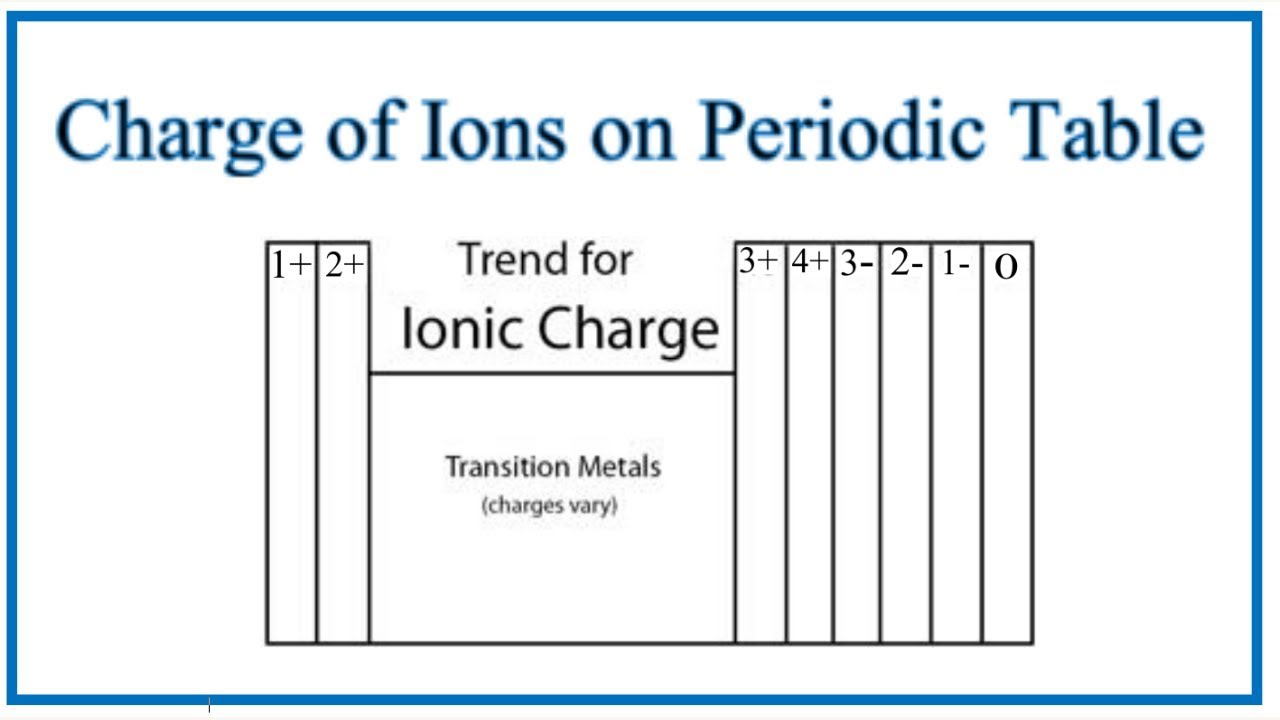

Metals in Groups 1, 2, and 13 = charges of 1+, 2+, and 3+
Charge on ions of the transition metals can vary which is why Roman numerals are often used to indicate their charge
ex. Copper (II) Oxide = copper ion has a charge of 2+
ex. Copper (I) Nitrate = copper ion has a charge of 1+
Non-metal Ions
Non-metals in Groups 15-17 have a negative charge and have the suffix “-ide”
ex. Nitride, Chloride, Bromide, Iodide
Elements in Group 17 = gain 1 electron → have a 1- charge
ex. Br-
Elements in Group 16 = gain 2 electrons → have a 2- charge
ex. O2-
Elements in Group 15 = gain 3 electrons → have a 3- charge
ex. N3-
Additionally, there are more polyatomic or compound negative ions (negative ions made up of more than one type of atom)
7 Common Polyatomic Ions
Ion | Formula and Charge |
|---|---|
Ammonium | NH4+ |
Hydroxide | OH- |
Nitrate | NO3- |
Sulfate | SO42- |
Carbonate | CO32- |
Hydrogen Carbonate | HCO3- |
Phosphate | PO43- |
4.1.4 Covalent Bonds
Covalent Bonds
Covalent Bonding occurs between 2 non-metals
Involves the electrostatic attraction between the nuclei of 2 atoms and the electrons of their outer shells
No electrons transferred, but only shared
2 atomic orbitals overlap and a molecular orbital is formed (see left side of image, “bonding”)
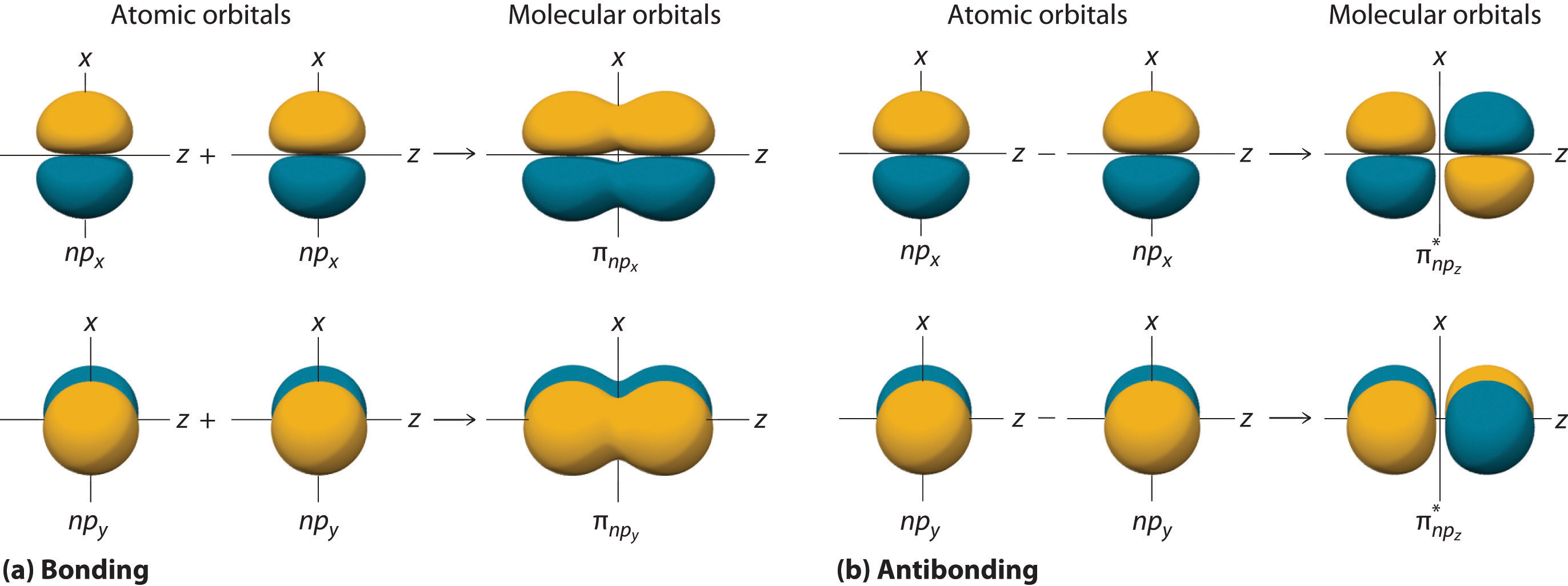
Covalent bonding occurs due to the fact that electrons are more stable when attracted to 2 nuclei rather than only 1
Why are they more stable?
Because sharing electrons allows each of the atoms to achieve an electron configuration similar to a noble gas (octet rule)
Usually, each atom provides one of the electrons in the bond
A covalent bond is represented by a short straight line between 2 atoms
ex. H-H
Note: Think about covalent bonds as electrons being in a constant state of motion, or “charge clouds” rather than an electron pair in a fixed position
Predicting Covalent Bonding
Use differences in electronegativity to predict whether a bond is either covalent or ionic
Electronegativity & Covalent Bonds
Electron density in diatomic molecules are shared equally
ex. H2, O2, Cl2
Difference in Electronegativity | Bond Type |
|---|---|
< 1.0 | Covalent |
1.0 - 2.0 | Polar Covalent |
> 2.0 | Ionic |
Coordinate Bonds
In simple covalent bonds, the 2 atoms involved share electrons
However, some molecules have a lone pair of electrons that can be donated to form a bond with an electron-deficient atom (an atom that has an unfilled outer orbital)
So both electrons are from the same atom
This type of bonding is called dative covalent bonding or coordinate bond
An example of a dative bond is in an ammonium ion
The hydrogen ion, H+ is electron-deficient and has space for two electrons in its shell
The nitrogen atom in ammonia has a lone pair of electrons which it can donate to the hydrogen ion to form a dative covalent bond
Multiple Bonds
Non-metals are able to share more than one pair of electrons to form different types of covalent bonds
Sharing electrons in the covalent bond allows each of the 2 atoms to achieve an electron configuration similar to a noble gas
This makes each atom more stable
It is not possible to form a quadruple bond as the repulsion from having 8 electrons in the same region between the two nuclei is too great
Type of Covalent Bond | # of electrons shared |
|---|---|
Single (C - C) | 2 |
Double (C = C) | 4 |
Triple (C ≡ C) | 6 |
Bond Length & Strength
Bond Energy
Bond Energy = The energy required to break one mole of particular covalent bond in the gaseous states (in units of kJ mol-1)
The larger the bond energy, the stronger the covalent bond is
Bond Length
Bond length = internuclear distance of two covalently bonded atoms
It is the distance from the nucleus of one atom to another atom which forms the covalent bond
The greater the forces of attraction between electrons and nuclei, the more the atoms are pulled closer to each other
This decreases the bondlength of a molecule and increases the strength of the covalent bond
Triple bonds are the shortest and strongest covalent bonds due to the large electron density between the nuclei of the two atoms
This increase the forces of attraction between the electrons and nuclei of the atoms
As a result of this, the atoms are pulled closer together causing a shorter bond length
The increased forces of attraction also means that the covalent bond is stronger

Triple bonds are the shortest covalent bonds so they are the strongest
4.1.5 Bond Polarity
When two atoms in a covalent bond have the same electronegativity the covalent bond is nonpolar (In other words, the difference in electronegativity = 0)
When two atoms in a covalent bond have different electronegativitiesthe covalent bond is polar and the electrons will be drawn towards the more electronegativeatom
As a result of this:
The negative charge center and positive charge center do not coincidewith each other
This means that the electron distributionis asymmetric
The lesselectronegative atom gets a partial charge of δ+ (deltapositive)
The moreelectronegative atom gets a partial charge of δ- (deltanegative)

The greater the difference in electronegativity the more polar the bond becomes
Dipole moment
The dipole moment is a measure of how polar a bond is
The direction of the dipole moment is shown by the following sign in which the arrow points to the partially negatively charged end of the dipole:

4.1.6 Lewis Structures
Lewis structures are simplified electron shell diagrams and show pairs of electrons around atoms.
A pair of electrons can be represented by dots
 The Octet Rule = The tendency of atoms to gain a valence shell with a total of 8 electrons
The Octet Rule = The tendency of atoms to gain a valence shell with a total of 8 electrons
Steps for drawing Lewis Structures
Count the total number of valence
Draw the skeletal structure to show how many atoms are linked to each other.
Use a pair of crosses or dot/cross to put an electron pair in each bond between the atoms.
Add more electron pairs to complete the octets around the atoms ( except H which has 2 electrons)
If there are not enough electrons to complete the octets, form double/triple bonds.
Check the total number of electrons in the finished structure is equal to the total number of valenceelectrons
Incomplete Octets
For elements below atomic number 20 theoctet rule states that the atoms try to achieve 8 electrons in their valence shells, so they have the same electron configuration as a noble gas
However, there are some elements that are exceptions to the octet rule, such a H, Li, Be, B and Al
H can achieve a stable arrangement by gaining an electron to become 1s2, the same structure as the noble gas helium
Li does the same, but losing an electron and going from 1s22s1 to 1s2 to become a Li+ ion
Be from group 2, has two valence electrons and forms stable compounds with just four electrons in the valence shell
B and Al in group 13 have 3 valence electrons and can form stable compounds with only 6 valence electrons
Unit 4.2 Resonance, Shapes, and Giant Structures
4.2.1 Resonance Structures
Some atoms or elements have structures that don’t seem to fit with what you would expect their typical Lewis Structure to be
This can be explained by the delocalization of electrons
Delocalized electrons = electrons in a molecule, ion, or solid metal that are not permanently associated with one atom or covalent bond
Example:
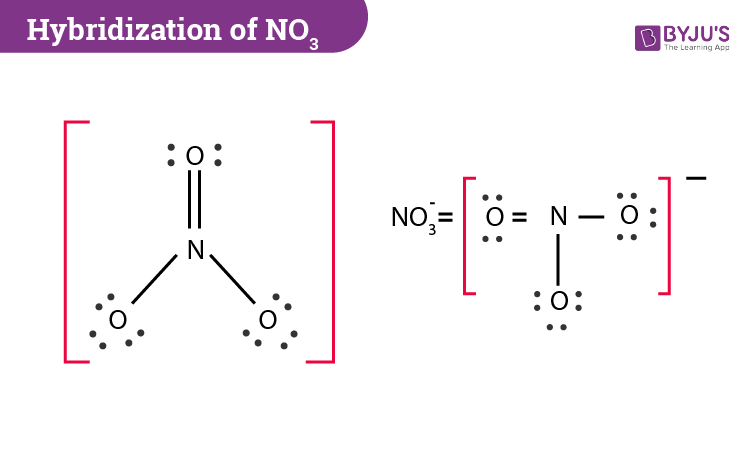
Nitrate (V) Ion
A molecule with 1 double bond and 2 single bonds
It has 3 possible Lewis Structures where the double bond moves around and is with each of the three oxygens

Since there are different possibilities, these Lewis Structures are also called Resonance Structures
So you would expect each of these Resonance Structures to have explicitly 1 double bond and 2 single bonds, right?
That’s not the case, however, because studies of the electron density and bond length show that all 3 bonds are equal in length
In fact, the electron density is spread evenly between the three oxygen atoms
The actual bond length for all of them is somewhere between a single and a double bond
The actual structure is something between all the resonance structures and is called a resonance hybrid
Resonance Structures of the Nitrate (V) Ion
Steps to Determine a Lewis Structure:
Count the # of Valence Electrons
Consider the Charge
Add more electrons for negative charges
Subtract electrons for positive charges
N + 3O + 1
5 + (3 × 6) +1
= 24 electrons
Draw a Skeleton
Put single bonds between atoms first
Generally, the least electronegative atom goes in the center
On the ends, put Hydrogens (because Hydrogen can only have 2 valence electrons and can only bond once) and Halogens
Subtract Skeletal Electrons from Valence Electrons
Use the remaining electrons to create Lone Pairs
OR additional bonds (double/triple) as needed
Remember the overall goal is to satisfy the Octet Rule
3 structures are possible for Nitrate (V) Ion, with 1 double and 2 single bonds
The negative charge is distributed throughout the ion and is depicted with the negative sign outside of the resonance brackets
Electron pairs rapidly oscillate between different positions, never really staying a single or a double bond for long
Criteria for forming resonance hybrid structures: molecules must have a double bond that is capable of migrating from one part of a molecule to another
In other words, when there are adjacent atoms with equal electronegativity and lone pairs of electrons that can move to another position in order for the double bonds to be in other positions
Ex. Carbonate Ion, Benzene, Ozone, and the Carboxylate Anion
4.2.2 Shapes of Molecules
VSEPR (Valence Shell Electron Pair Repulsion) Theory = A theory that predicts molecular shape and the angles between bonds based on the concepts:
All electron pairs and all lone pairs arrange themselves as far apart in space as possible
Lone Pairs repel more strongly than bonding pairs
Multiple bonds (double/triple) behave as single bonds
Domains = The regions of negative cloud charge
Steric Number (SN) = # of atoms + # of Lone Pairs around the central atom (same concept as Domains)
Can also be denoted as:
A = Central Atom
B = Bonded Pair
E = Lone Pair
ex. SN = 2 → AB2
ex. SN = 3 → AB3
ex. SN = 3 (but one of the domains is a lone pair) → AB2E1

Steric Number = 2
If SN = 2, then the angle between bonds is 180°
SN = 2 → AB2
Molecular Geometry = “Linear”
ex. BeCl2, CO2, HC≡CH

Steric Number = 3
If SN = 3, then the angle between the bonds is 120°
SN =3 → AB3
Molecular Shape = “Trigonal Planar”
ex. BF3 and CH2CH2 and CH2O


AB2E1
If one of the electron domains is a lone pair, then the bond angle is slightly less than 120° since lone pairs repulse more, pushing against the other two bonding pairs closer together
ex. SO2
Molecular Geometry = “Bent Linear”


Steric Number = 4
If SN = 4, then the angle between bonds is 109.5°
E.g. CH4, NH4+
SN = 4 → AB4
Molecular Geometry = “Tetrahedral”

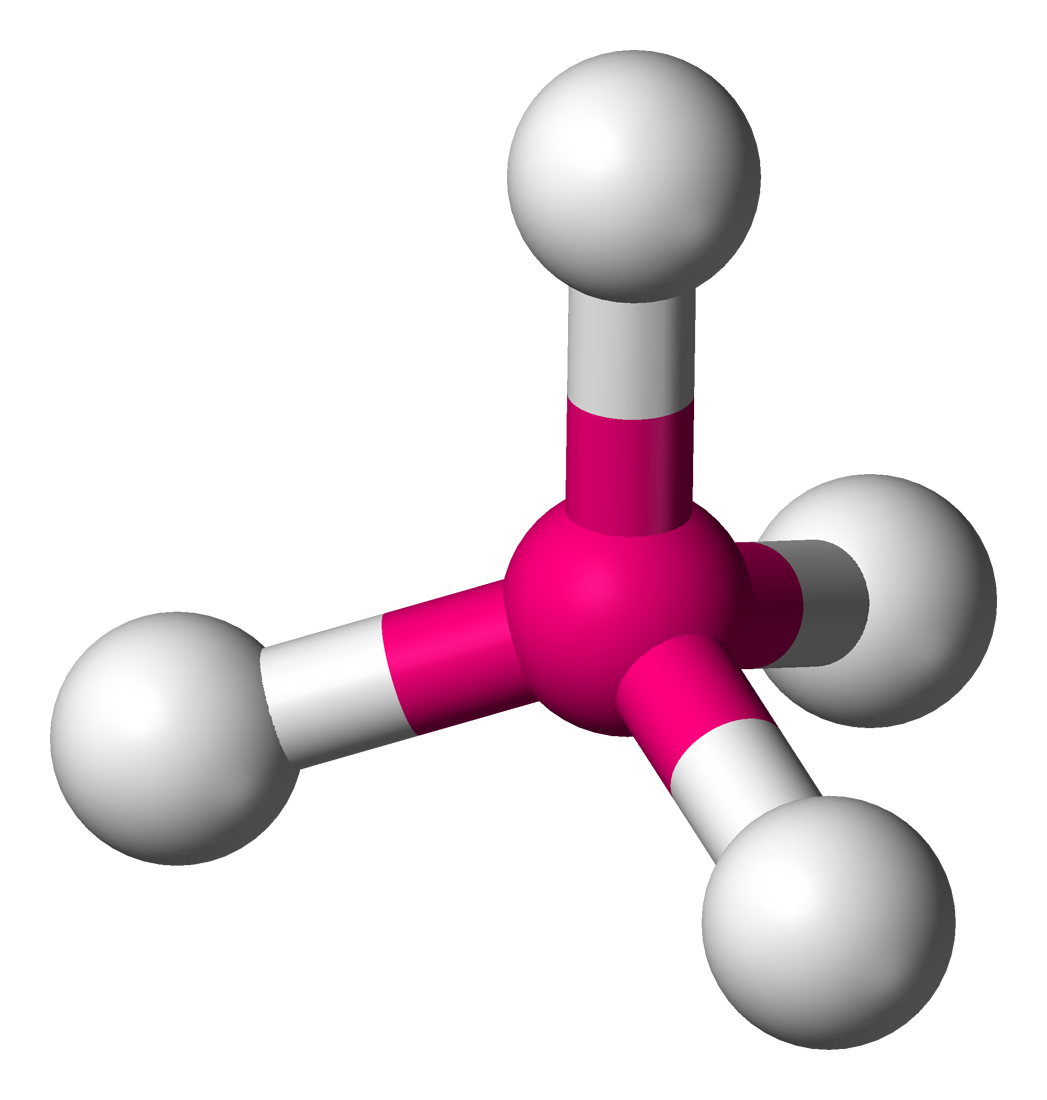
AB3E1
If one of the electron domains is a lone pair, the bond angle is slightly less than 109.5° due to increased lone pair repulsion
ex. NH3
Molecular Geometry = “Trigonal Pyramidal”


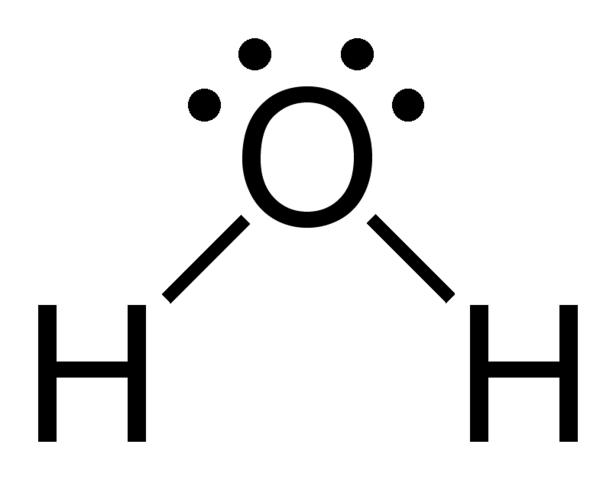
AB2E2
If 2 electron domains are lone pairs, bond angle also less than 109.5°
ex. H2O
Molecular Geometry = “Bent”

Summary


4.2.3 Predicting Shapes & Bond Angles
Draw Lewis Structure
Determine the number of bonding (B) and Lone Pairs (E) around the central atom (A)
Apply VSEPR Rules
Deduce shape and bond angle
4.2.4 Molecular Polarity
Bond Polarity ≠ Molecular Polarity
Previously, you learned that bond polarity was determined by the difference in electronegative felt between two bonded atoms
However, now you can determine if a molecule is polar or not
Consider:
The polarity of each bond in the molecule
How the bonds are arranged in the molecule
Note: Some molecules have polar bonds, yet are overall not molecularly polar since the polar bonds in the molecule are arranged in a way that the individual bond dipole moments cancel each other out
ex. CH3Cl vs. CCl4
CH3Cl
Has 4 polar covalent bonds that don’t cancel each other out
This means the molecule is polar overall
The overall dipole moment is pointing towards the electronegative chlorine atom
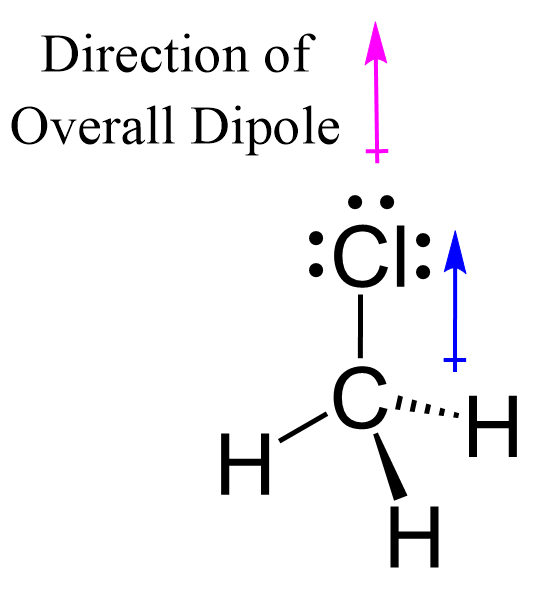
CCl4
Also has 4 polar covalent bonds, BUT the individual bond dipole moments cancel each other out
So CCl4 is a nonpolar molecule

4.2.5 Giant Covalent Structures
Giant Covalent Structures
Covalent Lattices
Covalent Bonds = Bonds between nonmetals in which electrons are shared between atoms
Giant Covalent Substances = Sometimes, a substance can't bond like a regular molecule. Instead, the bonds between atoms continue forever, forming a big lattice. There are no separate molecules in this situation, and all the nearby atoms are connected by covalent bonds.
ex. C
Allotrope = Different atomic or molecular arrangements of the same element in the same physical state
Graphite, diamond, buckminsterfullerene and graphene are allotropes of carbon
Giant Covalent Structures Examples
Diamond
Diamond is a giant lattice of carbon atoms
Each carbon is covalently bonded to 4 others in a tetrahedral geometry with a bond angle of 109.5°
This results in a giant lattice with strong bonds in all directions and causes diamond to be the hardest known substance
Graphite
Each carbon atom is bonded to 3 others in a layered structure of hexagons with a bond angle of 120°
The spare electron is delocalized and moves around in the space between the layers
All atoms in the same layer are held together by strong covalent bonds while the different layers are held together by weak intermolecular forces
![Graphite [SubsTech]](https://www.substech.com/dokuwiki/lib/exe/fetch.php?w=&h=&cache=cache&media=graphite_structure.png)
Buckminsterfullerene
Contains 60 carbon atoms
Each atom is bonded to 3 others by single covalent bonds
The fourth electron is delocalized so the electrons can migrate throughout the structure
This allows for the structure to be a semi-conductor
Has the same shape as a soccer ball, so it is nicknamed the football molecule

Graphene
Some substances infinitely covalent bond only in two dimensons, forming only layers
ex. Graphene
Graphene is a single layer of carbon atoms bonded in a repeating hexagonal pattern
It is so thin, 1 million times thinner than paper, that Graphene is actually considered 2D

Properties of Giant Covalent Structures
As always, different structures and bonding types have different effects on the physical properties of substances (ie. melting/boiling points, electrical conductivity, and solubility)
Covalent Bonding & Giant Covalent Lattice Structures
Giant Covalent Lattices:
Very High melting and boiling points
Large # of covalent bonds
A lot of energy is needed to break the lattice
Can be hard or soft
Hard (difficult to break their 3D network of strong covalent bonds)
Diamond
Silicon (IV) Oxide
Soft (forces between carbon layers are weak)
Graphite
(Graphene is strong, flexible, and transparent, making it a very useful material)
Insoluble in water (Most)
Do NOT conduct electricity (Most)
The some that do have delocalized electrons:
Graphite
Graphene
Buskminsterfullerene (semi-conductor)
Unit 4.3 Intermolecular Forces & Metallic Bonding
4.3.1 Types of Intermolecular Forces
Intermolecular Forces (IMF)
Forces of attraction between molecules that hold them together
3 main types:
Dispersion forces
Dipole-dipole
Hydrogen bonding
Dispersion Forces
Electrons in constant motion in an atom
Electron cloud dispersion can be asymmetrical at any given point in time, creating an instantaneous dipole
Attraction between partial negative and positive instantaneous dipoles form a dispersion force
Strength of dispersion forces increases with difference in electronegativity and electron cloud movement
Key Facts about Dispersion Forces
Present in all atoms and molecules
Weakest IMF
Strength depends on the number of electrons
More electrons = stronger temporary/instantaneous dipole
Exception: dispersion forces can be stronger than dipole-dipole if the atom is large enough
Molecules composed of only C and H can only have dispersion forces
Dipole-dipole Attractions
Attractive force between molecules with permanent dipoles
Stronger than dispersion forces
Only for small molecules with the same number of electrons
Hydrogen Bonding
Strongest IMF
Special type of dipole-dipole attraction
Conditions for hydrogen bonding to occur:
A species with a very electronegative atom (O, N, or F) that has a lone pair of electrons
A hydrogen attached to the O, N, or F
Hydrogen becomes partially positively charged and can form a bond with the lone pair on another molecule
Hydrogen Bond Donors and Acceptors
Every hydrogen bond has two components
A molecule can be both the donor and acceptor, able to hydrogen bond with itself
Hydrogen bond acceptor only requires an available lone pair, not a hydrogen atom
Hydrogen Bonding in Water and Ammonia
Water can form a maximum of two hydrogen bonds per molecule
Ammonia can form a maximum of one hydrogen bond per molecule
Number of hydrogen bonds possible is restricted by the number of
4.3.2 Deducing Intermolecular Forces
The structure and chemical formular of the molecules will indicate the types of intermolecular forces present
Structure and Symmetry → Is molecule polar or not? (See Section 4.2.4)
Chemical Formula → How electronegative are the elements in the molecule?
Helps to tell you polar bonds
Also tells you if hydrogen bonds are possible when there is N, O, or F
4.3.3 Properties of Covalent Compounds
Types of intermolecular forces indicate physical properties of molecular covalent compounds (melting/boiling point, solubility, and conductivity)
Melting and Boiling Point
Changing the state means overcoming the intermolecular forces
The stronger the forces, the more energy is needed to break the attraction between molecules
Substances with low melting and boiling points = “volatile”
As the intermolecular forces increase in strength:
The size of the molecule increases
The polarity of the molecule increases
Solubility
“Like dissolves like” = non-polar substances dissolve in non-polar solvents while polar substances dissolve in polar solvents
However, as the size of a covalent molecule increases in size at a certain point, their solubility can decrease
This is because the polar part of the molecule remains the smaller part of the overall structure (In other words, the ratio of polar to non-polar decreases)
Ex. alcohols (ethanol is soluble yet hexanol isn’t)
Giant Covalent substances don’t dissolve in any solvents
This is because the energy required to overcome their strong covalent bonds from the lattice structure is too great
Conductivity
Usually, covalent substances can’t conduct electricity in solid or liquid states since they don’t have any free-moving charged particles
Only in some cases, polar covalent molecules can ionize and conduct electricity
Other exceptions are Giant Covalent Structures and they can conduct electricity because they have delocalized electrons (the free-moving charged particles required for conductivity) (See Section 4.2.5)
4.3.4 Metallic Bonding
Metallic Bonding
Metal atoms tend to pack together in lattice structures
This causes their outer electrons to be able to move freely throughout the entire structure = “delocalizes electrons”
Once their valence electrons are delocalized, the metals gain a positive charge, which repel each other, keeping the entire structure neatly arranged in a lattice
Metallic Bonding involves strong electrostatic forces of attraction between the metal centers and delocalized electrons
Properties of Metals
Metals are malleable
This is because when a force is applied, the metal layers can slide over each other (the attractive forces between the metal ions and the delocalized electrons act in all directions)
So, when the layers slide, the metallic bonds can re-form in a new shape and the lattice is not broken
Metallic compounds are strong and hard
Due to the strong attraction between the metal cations suspended in a sea of delocalized electrons
This also causes metals to have a high melting and boiling point
Conductivity
Unlike non-metals, metals are able to conduct electricity when in the solid or liquid state
Because in both states, they have mobile electrons that can move around and conduct electricity (remember: electric current = flow of electrons)
Strength of Metallic Bonds
Not all metallic bonds have equal strength; there are several factors that affect it:
Charge on the Metal Ion
The greater the charge on the metal ion,
→ the greater number of electrons in the sea of delocalized electrons
→ the greater the charge difference between ions and electrons
→ the greater the electrostatic attraction
→ the stronger the metallic bond
Radius of the Metal Ion
Metal ions with a smaller ionic radii exert a greater attraction on the sea of delocalized electrons
→ requires more energy to break
→ stronger metallic bond
4.3.5 Trends in Melting Points of Metals
An increase in the strength of electrostatic attraction is caused by:
Increasing the # of delocalized electrons in each metal atom
Increasing the positive charges on the metal centers in the lattice structure
Decreasing the size of the metal ions
Melting Points of Metals Across a Period
Ex. Compare the electron configuration of sodium, magnesium, and aluminum and observe the # of valence electrons (do they increase or decrease?)
Na = 1s22s22p63s1
Mg = 1s22s22p63s2
Al = 1s22s22p63s23p1
Since aluminum ions are smaller in radius than magnesium or sodium ions
So considering that aluminum has the most electrons AND has the smallest radius, it has a stronger metallic bonding → higher melting point
So as you go across a period, the metallic bonding is stronger and the melting points increase
Melting Points of Metals Down a Group
As you go down a periodic group, the size of the metal cations increase, thus decreasing the attraction between the negative valence electrons and the positive metallic lattice, so the melting point decreases
4.3.6 Alloys & their Properties
Alloys = mixtures of metals (the metals are mixed together physically but are not chemically combined)
Alloys can also be a mixture of metals and non-metals (ex. with carbon)
The different metal ion mix is spread evenly throughout the lattice (not clumped together) and are bound together by their delocalized electrons
Alloys are able to form due to the fact that metallic bonds are non-directional by nature
So why are Alloys made?
They have distinct and desirable properties since the cations are structured differently in the lattice
Alloy Properties
Greater strength,
Harder
Since the mixture of atoms in an alloy are different sizes, this distorts the regular arrangement of cations
So the layers in a lattice structure have a more difficult time sliding over each other, causing the alloy to be harder than a pure metal
Higher resistance to corrosion/extreme temperatures
Common Alloys & their Uses
Alloy | Elements | Properties | Uses |
|---|---|---|---|
Brass | copper + zinc | strong resistant to corrosion | door handles, hinges, metal instruments |
Steel | iron + carbon + others (chromium, vanadium, and molybdenum, etc.) | very strong | construction, bridges, cars |
Solder | lead + tin | low melting point | joining metals in electrical circuits and in jewelry |
Bronze | copper + tin | hard strong resistant to corrosion | medals, sculptures, ship fittings |