Thermochemistry & Thermokinetics
Calorimeters are closed systems
ΔH= -q
Thermochemistry is the study of energy changes that accompany physical or chemical changes in matter
Thermal energy is the energy available from a substance as a result of the motion of its molecule
Heat (q - units Jules) is transferred between the system and surroundings
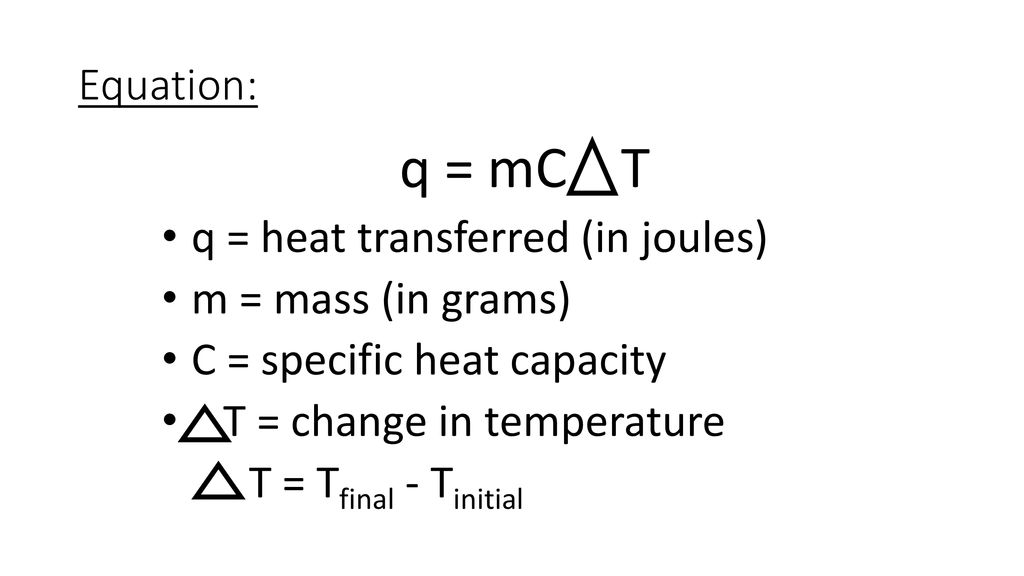
Usually, the specific heat capacity is that of water (4.18 J/gxºC)
Enthalpy: the measure of bond stability in a system
systems favour lower enthalpy (more stable)
Units —> Kilojules/mole
Exothermic - releases energy to surroundings, spontaneous (-ΔH)
+ energy on the product side
increase in temperature
Endothermic - gains energy from surroundings, nonspontaneous (+ΔH)
+ energy on the reactant side
decrease in temperature
Spontaneous - starts without continuous outside influence
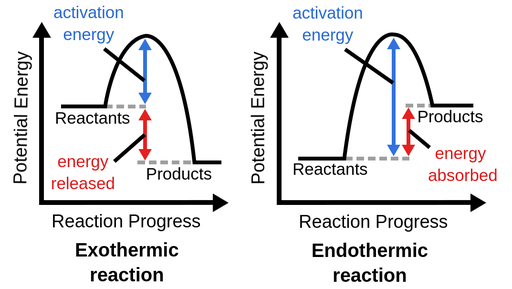
Enthalpy change is the difference in enthalpies of reactants and products during a change.

The enthalpy change per mole of a substance in the reaction is called the molar enthalpy
ΔH = n x ΔHx
ΔH =. enthalpy (kJ)
ΔHx= molar enthalpy(kJ/mol)
n = moles (mol) of limiting
In a calorimetry experiment, 3 assumptions are made:
no heat is transfered between the calorimeter and the outside environment
any heat absorbed or released by the calorimeter materials, like the container, is negligible
Dilute solutions have the specific heat capacity of water (4.18 J/gxºC)
Hess’ Law
States that the value of ΔH for any reaction that can be written in steps is the sum of the individual values of ΔH for each step.
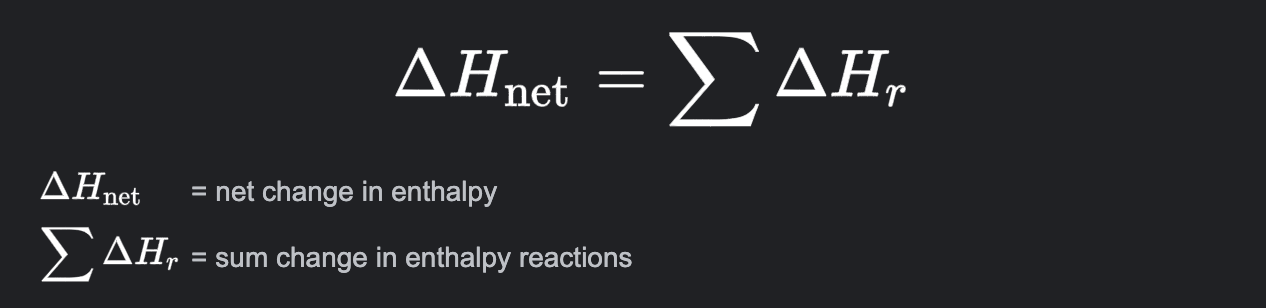
If a chemical reaction is reversed, the sign of ΔH changes
if a chemical reaction i multiplied or divided, then ΔH is altered the same way
Chemical Kinetics
chemical kinetics is the study of ways to make chemical reactions go faster or slower
reaction rate is the speed at which a chemical change occurs
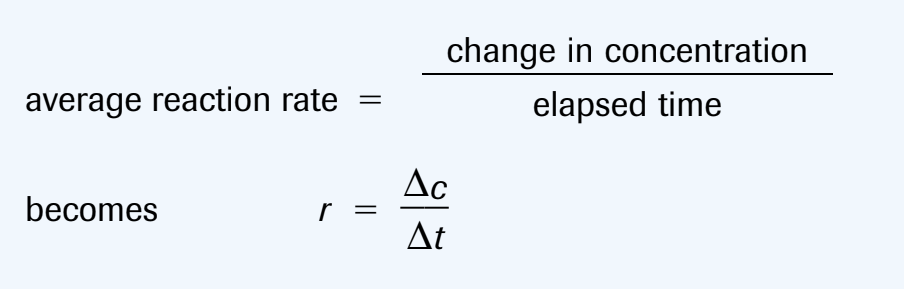
On a graph, the average rate of reaction over a time period is the absolute value of the slope of the secant
The instantaneous rate of reaction is the slope of the tangent
(-) sign indicate a rate of consumption of the reactants
(+) sign indicate a rate of production of the products
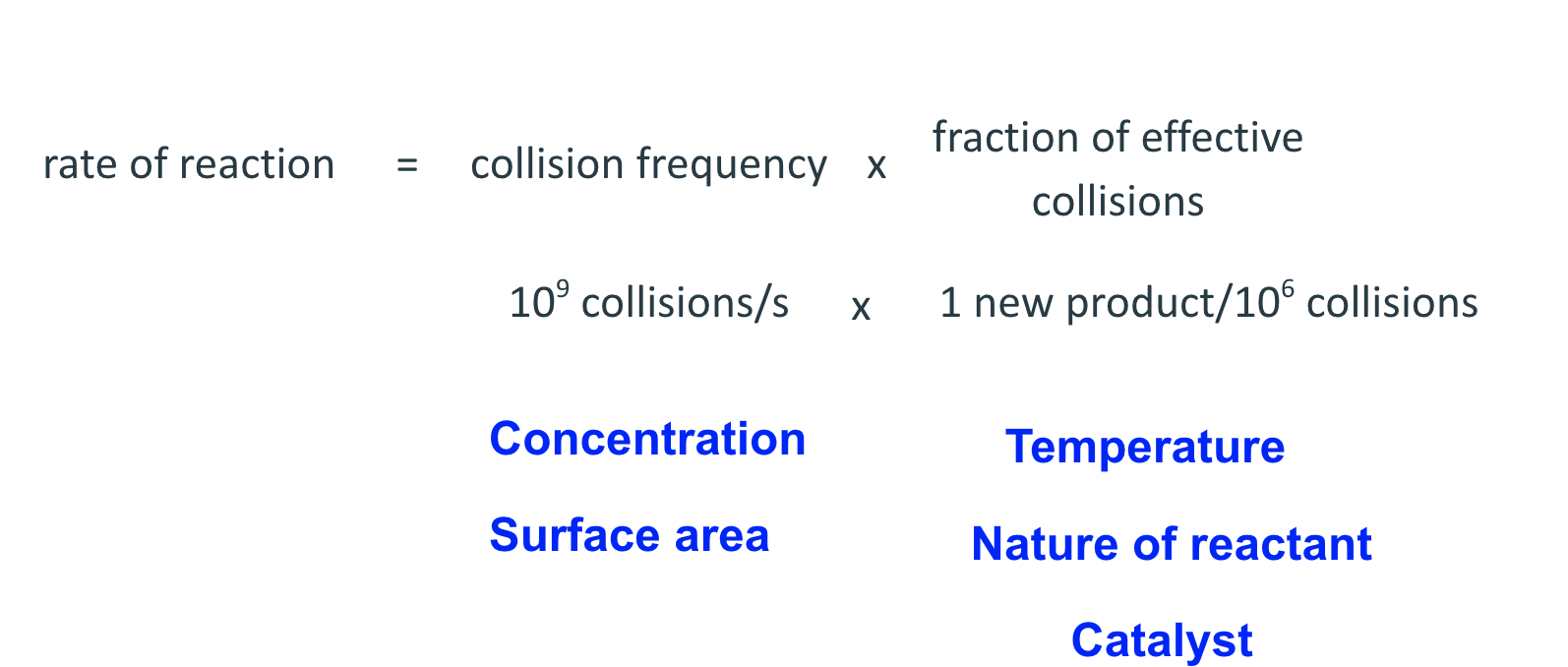
Factors that affect rates:
nature of reactants :
concentration of reactants: if the initial concentration of a reactant is increased, then the reaction rate increase
Temperature: Chemical reactions typically occur faster at higher temperatures
presence of catalyst: speeds up a reaction by providing an alternative route, requiring a lower activation energy, EA (large k value)
surface area: reaction rates increases proportionally with an increase in surface area
Rate is always proportional to the product of the initial concentration of the reactants where these concentration are raised to some exponent
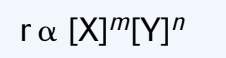
 (k is the rate constant - determined through calculations and only at specific temperatures)
(k is the rate constant - determined through calculations and only at specific temperatures)m and n are exponents that describe the relationship between rate and initial concentration and can only be determined empirically.
can be all real numbers (0, fractions…)
don’t have to be equal to the coefficients in the balanced equation.
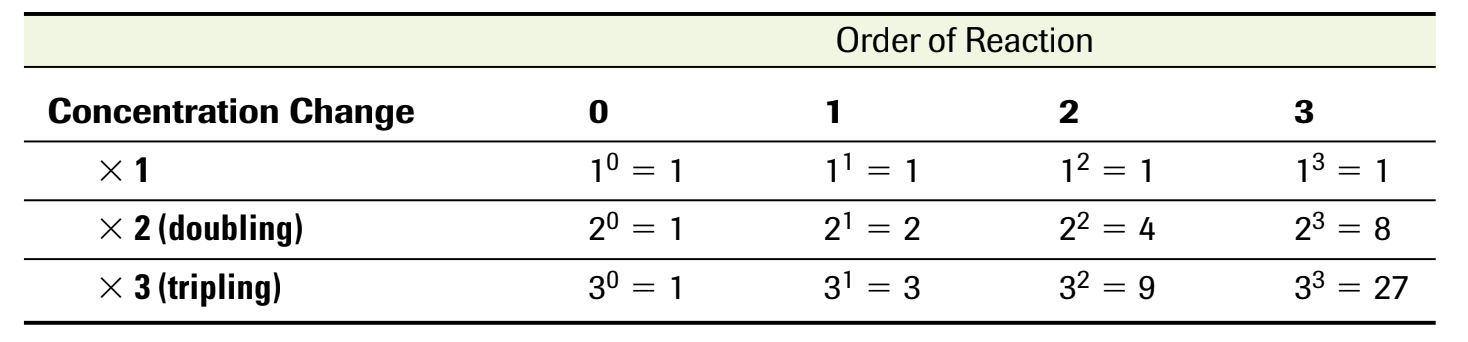
The order of reaction is the value of the exponent that describe the initial concentration dependence of a reactant
overall order of reactant is the sum of the exponents of the rate law equation
ex. r= k[NO2]1[F2]1
order with respect to NO2 is 1
order with respect to F2 is 1
overall order is 2 (1+1)
Rate Law Using Initial Concentrations
The rate law of a reaction is based on the initial concentrations of the reactants, the rate of the disappearance of products and the appearance of the products and requires experimental data. Arrhenius constant, k, is based on the activation energy for a reaction and the temperature.
Rate = k[A]^x[B]^y[C]^x
The value of the exponent is based on how the reactants affect the rate. Larger exponent means the reactant concentration changes more. To find the exponent value, find what happens when a concentration is doubled.
Rate law can be expressed using concentration changes over time. There are zero, first, and second order rate laws that each have the roan equation and graph.
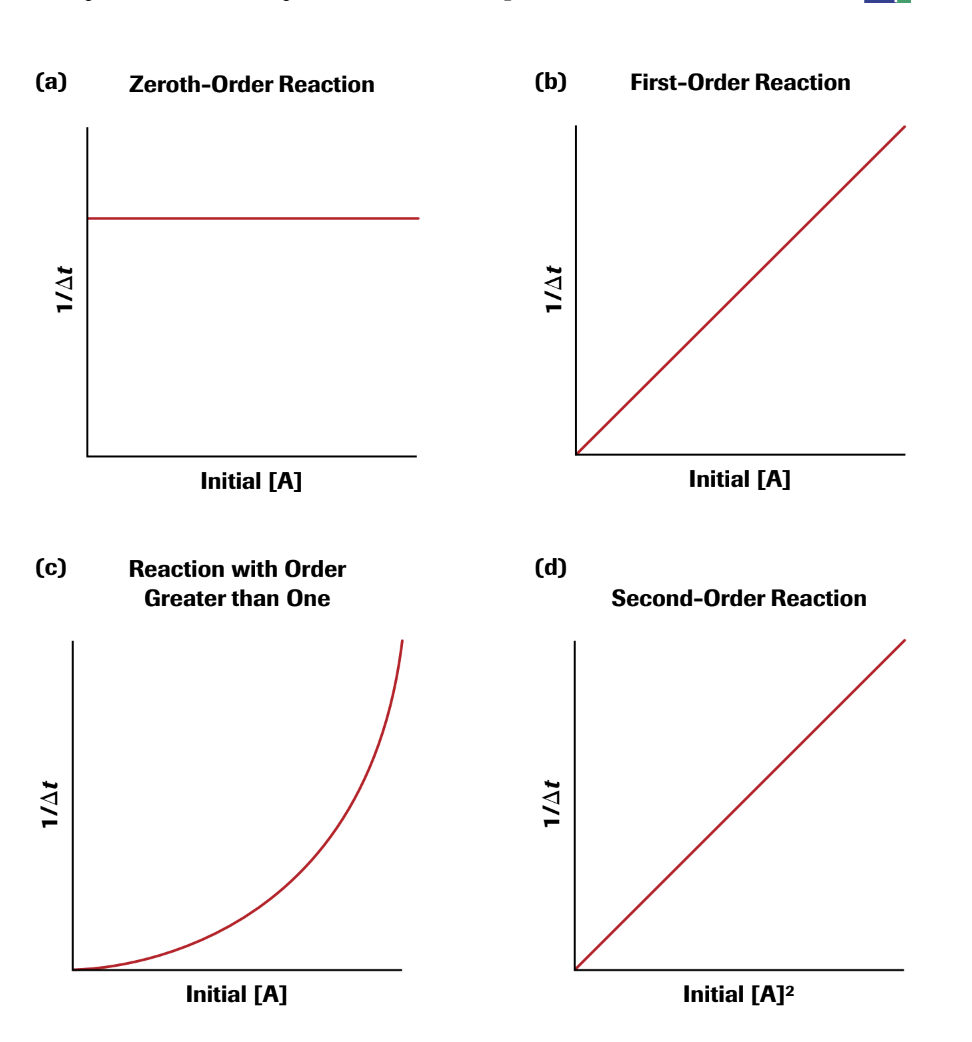
Zero-Order Rate Laws
In zero order, the rate is always the same at a given temperature and doesn't change based on reactant concentration.
Rate = k
The graph is a straight line equal to -k and concentration vs. time.
First- Order Rate Laws
First order rate law is based on the concentration of one reactant to the first power.
Rate = k[A]
The graph is the natural log of [A] vs. Time. This creates a straight line with a slope of -k and y-interest of ln[A] at time 0.
Collision Theory
Collision theory states reactions only occur when chemicals collide with each other with sufficient energy (activation energy).
They can react more if there's a higher concentration of aqueous or gaseous substances, or a high surface area of a solid substance.
Stirring can sometimes speed up a reaction. If the mixture is heterogeneous, not all parts of a mixture is identical, then stirring will mix it more and speed it up. If the mixture is homogeneous, where the mixture is completely identical, then stirring will not help because it is already mixed and even.
Reaction rate increases with temperature because molecules are moving faster and have a higher likelihood to collide with other molecules at a sufficient speed.
Molecules will only react if they collide with the correct orientation.
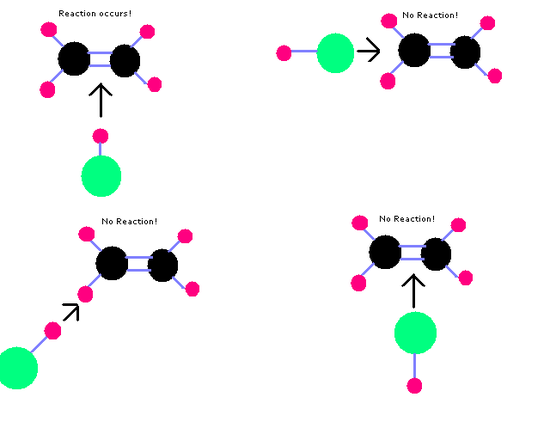 Reaction Mechanisms
Reaction Mechanisms
Some reactions occur in multiple steps rather than in one step and the overall balanced reaction is the sum. The in between steps are called elementary steps.
Example: The rate for elementary steps can be determined by setting the concentration of a substance to the power of it’s coefficient
I. A + A ⇌ X (fast) Rate=k[A]^2
II. X + B → C + Y (slow) Rate=k[X][B]
III. Y + B → D (fast) Rate=k[Y][B]
X and Y are intermediates, they are created in one step and consumed in another, so they cancel out.
If an elementary step has only one reactant, it is unimolecular. If it has two reactants (even if they are the same reactant, like in step l), it is considered bimolecular.
The slowest step is considered the rate-determining step because the speed of the reaction cannot exceed the slowest step. This step determines the rate law for the entire reaction.
Step II has an intermediate, which is not written in the reaction, and it is produced in step I, so X can be replaced with [A]^2. The rate law for step two and the overall reaction becomes Rate=k[A]^2[B].
a multi - step reaction can be written as a reaction energy profile diagram
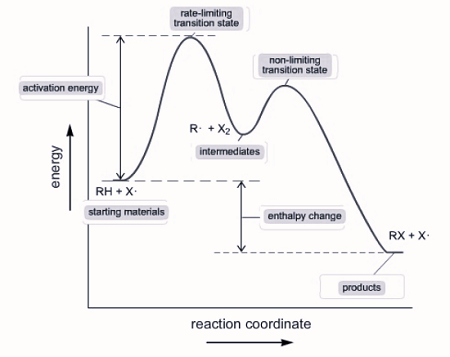
Catalysts
Catalysts are added to a reaction to speed it up without being consumed. Catalysts are present in the beginning and end of elementary steps and cancel out.
For the steps in the reaction A + B → C
I. A+ X→ Y
II. B + Y → C + X
In this reaction, x is the catalyst and Y is the intermediate