Paper chromatography of amino acids
Chromatography can be used to separate substances based upon their differential solubility in a solvent (mobile phase) which can be a liquid or a gas. The substances that are more soluble in the solvent (show a higher affinity for the mobile phase than the stationary phase) are carried further by the solvent through the stationary phase. The stationary phase may be paper or the silica gel coating on a TLC plate.
Paper chromatography using an organic solvent can be used to separate and identify amino acids in a mixture (usually obtained from the hydrolysis of a protein). Amino acids can be separated using this technique because they each have a different R group which alters the solubility of the amino acid in a particular solvent (R groups may be polar, non-polar, or charged).
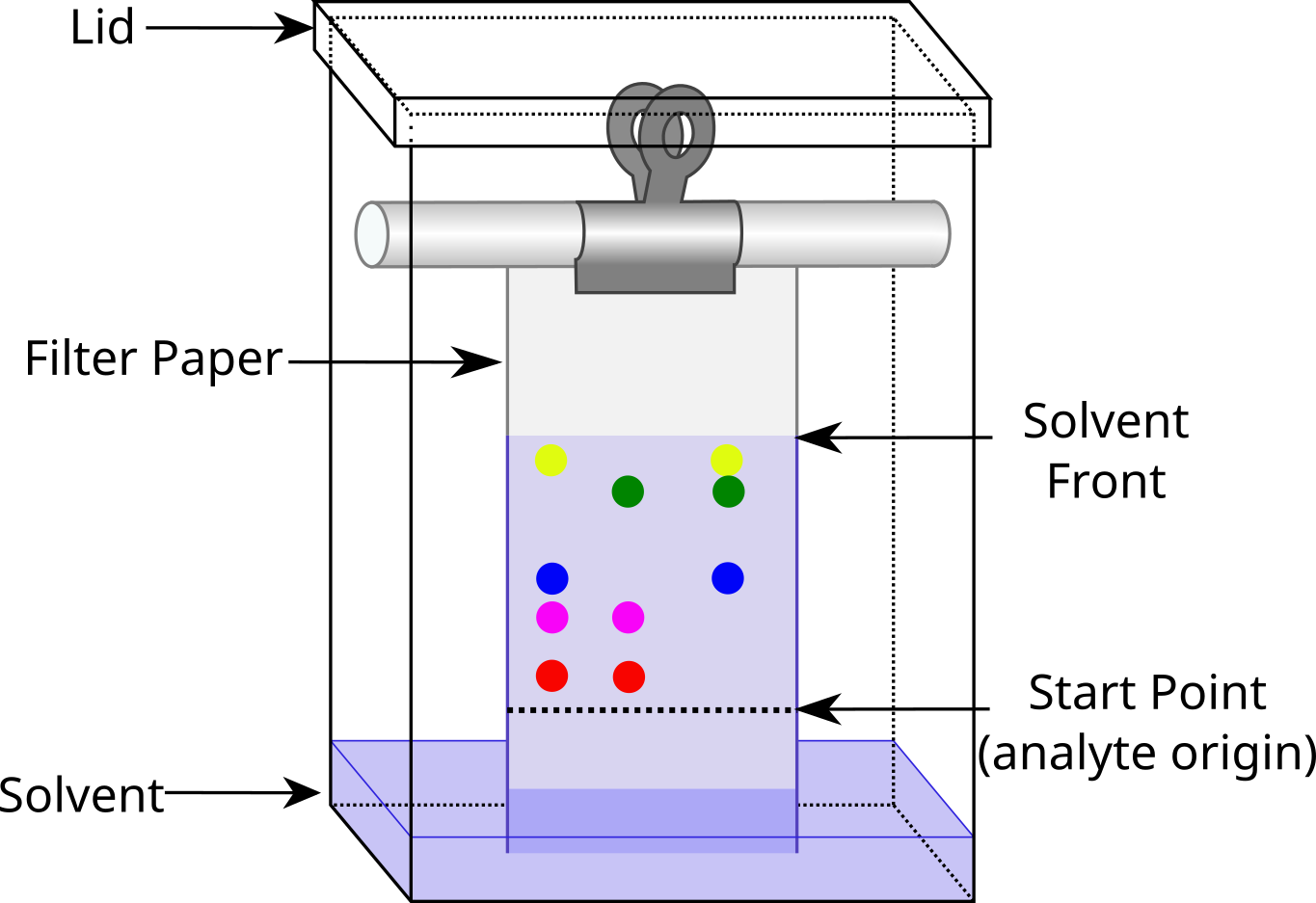
The start line should be drawn in pencil because pen ink will dissolve in the solvent and interfere with the results.
A capillary tube should be used to apply multiple drops to 1 spot, letting them dry before each application, so that each spot is concentrated ensuring that the separated amino acids are visible.
The paper must not touch the sides to ensure that the solvent doesn’t run faster along the sides resulting in an uneven solvent front.
The start line should be above the solvent so that the spots don’t dissolve out of the paper and into the solvent.
The chromatogram should run for as long as possible (2cm below the end of the paper) so that there is a greater separation between spots resulting in more distinct spots and more accurate measurements.
The paper should be sprayed with a locating agent called ninhydrin which stains the amino acids purple except proline which is stained yellow.
The chromatogram should be kept and sprayed in a fume cupboard as the solvent and ninhydrin are irritant.
Only handle the chromatogram with gloves as oils and amino acids on skin may interfere with the chromatogram.
The solvent front should be drawn immediately once the chromatogram is done as the solvent will evaporate quickly.
To aid in the identification of amino acids, the mixture of amino acids and known standards (solution containing only 1 type of amino acid) are run alongside each other on the same chromatogram. Calculating Rf values and comparing them with known values also helps to identify amino acids. Rf values can be calculated by dividing the distance the substance (mino acid) has travelled by the distance the solvent has travelled (solvent front)