AQA GCSE Chemistry Trilogy: Electrolysis
Electrolysis:
Electrolytes:
- Are ionic compounds
- Are molten
- Are dissolved in water
- Therefore, ions can move freely in the solution
Electrodes and Ions:
- Electrical energy from a direct current (DC) supply breaks down electrolytes
- Free-moving electrolytes are attracted to oppositely charged electrodes
- The anode is the positively charged electrode
- The cathode is the negatively charged electrode
Products of Electrolysis:
When ions reach an electrode, they gain or lose electrons. As a result, they form atoms or molecules of elements
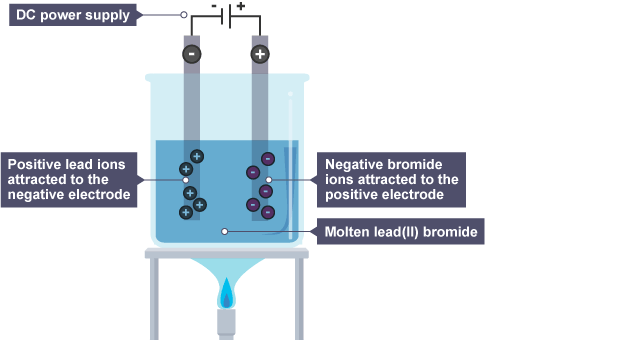
Molten lead bromide, PbBr2(l), is an electrolyte. During electrolysis -
- Pb2+ ions gain electrons at the cathode and become Pb atoms
- Br- ions lose electrons at the anode and become Br atoms, which pair up to form Br2 molecules
Electrolysis of Acified Water:
- Water is a poor conductor of electricity, but it does contain some hydrogen ions, H+, and hydroxide ions, OH-. These ions are formed when a small proportion of water molecules naturally dissociate (break up)
- If water is acidified with a little dilute sulfuric acid -
- H+ ions are attracted to the cathode, gain electrons and form hydrogen gas
- OH- ions are attracted to the anode, lose electrons and form oxygen gas
Electrolysis of Dissolved Ionic Compounds:
An electrolyte formed by dissolving an ionic compound contains -
- Hydrogen ions from the water, and positive ions from the compound
- Hydroxide ions from the water, and negative ions from the compound
At the cathode -
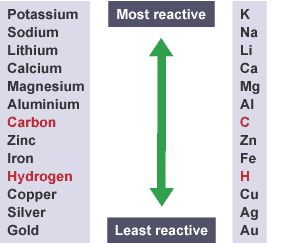
- Whether hydrogen or a metal is produced at the cathode depends on the position of the metal in the reactivity series.
- The metal is produced at the cathode if it is less reactive than hydrogen
- Hydrogen is produced at the cathode if the metal is more reactive than hydrogen
At the anode -
- Oxygen is produced (from hydroxide ions) unless halide ions (chloride, bromide or iodide ions) are present. In that case, the negatively charged halide ions lose electrons and form the corresponding halogen (chlorine, bromine or iodine).
Oxidation and Reduction:
Half-Equations:
- Used to represent the reaction that happens at an electrode during electrolysis
- Shows what happens when ions gain or lose electrons
- Electrons are shown as e-
- The number of atoms of each element must be the same on both sides
- The total charge on each side must be the same (usually zero)
- Cathode reactions -
- Positively charged ions gain electrons at the cathode
- Na+ + e- → Na
- Pb2+ + 2e- → Pb
- 2H+ + 2e- → H2
- Anode reactions -
- Negatively charged ions lose electrons at the anode.
- 2Cl- → Cl2 + 2e-
- 2O2- → O2 + 4e-