3.1.3 Lipids
Unit 1: Biomolecules
3.1.3 Lipids
Key Notes
Name: ____________________
Hydrocarbons | Molecules that only contain hydrogen and carbon atoms. |
Triglyceride | A lipid made of a glycerol molecule bonded to 3 fatty acid molecules. |
Fatty acids | A carboxylic acid group joined to a hydrocarbon tail. Tail can be of varying length. |
Saturated fatty acid | A fatty acid that does not contain any double bonds between carbon atoms. The maximum number of possible hydrogens are bonded to the fatty acid. |
Unsaturated fatty acid | A fatty acid that contains 1 or more double bonds between carbon atoms. |
Ester bond | The bond that forms between a glycerol and fatty acid in a condensation reaction. |
Condensation reaction | A reaction in which a bond is formed, releasing a water molecule. |
Phospholipid | A lipid made of a phosphate group, glycerol molecule and 2 fatty acids. |
Bilayer | A double-layered structure that forms from phospholipids, forming membranes in cells. The hydrophilic phosphate heads point outwards whilst the hydrophobic fatty acid tails point inwards. |
Hydrophilic | Attracts water. |
Hydrophobic | Repels water. |
Emulsion test | A test using ethanol, shaking and then water to identify the presence of a lipid. A positive result is a milky-white emulsion forming. |
- The elements contained in lipids are carbon, hydrogen and oxygen.
- Lipids are individual molecules, NOT polymers. They are not made up from repeating monomers.
- They are made of different individual molecules (glycerol, fatty acids, and in the case of phospholipids a phosphate group).
- Lipids are a large and varied group of non-polar molecules that are insoluble in water, but dissolve easily in organic solvents, like alcohols and acetone.
- They form the main part of cell membranes, are a source of energy (they store twice the amount of energy per gram compared to carbohydrates) They can also provide a waterproof layer, insulation and protection around major organs.
- Fats and oils are lipids. Fats are solid at room temperature and oils are liquid at room temperature.
![]() Triglycerides
Triglycerides
- Triglycerides are made up of one molecule of glycerol and 3 fatty acids attached to it.
- Glycerol has 3 hydroxyl groups (-OH), each of which can combine with a fatty acid forming a triglyceride.
- The formation of a triglyceride is a condensation reaction resulting in the production of 3 molecules of water.
- The bonds formed between the –OH groups of the glycerol and the -COOH of the fatty acids are called ester bonds (strong covalent bonds).
Diagrams of triglycerides: |
|
The formation of a triglyceride.
Triglycerides have many biological roles such as energy storage, insulation (electrical and heat) and protection. The structure of triglycerides are well suited to their functions:
Structure of triglycerides | Function of triglycerides |
They have a high ratio of energy storing carbon-hydrogen bonds to carbon atoms (long fatty acid tails) – contain lots of chemical energy | Energy storage. |
They have a low mass to energy ratio | Makes them good storage molecules because so much energy can be stored in a small volume (contain about twice as much energy per gram as carbs). |
They are large, non-polar molecules, insoluble in water. | They have no osmotic effect and do not affect the water potential in cells. They can clump together in insoluble droplets (hydrophobic tails facing inwards). |
Fatty Acids
- Fatty acids are organic compounds that have the general formula CH3(CH2)nCOOH , where n usually ranges from 2 to 28 and is always an even number.
|
- The general formula for a fatty acid can be written as RCOOH.
- Fatty acids are made up of a carboxylic acid group (-COOH) and a long hydrocarbon chain of varying length.
- Fatty acids are hydrophobic which means they repel water.
- All fatty acids have same basic structure, but their hydrocarbon tails can vary (variable ‘R’ group).
- The hydrocarbon chain of a fatty acid can be unsaturated or saturated.
- The thing that fatty acids are saturated with is hydrogen; in a saturated fat, as many hydrogen atoms as possible are attached to the carbon skeleton.
Saturated fatty acid |
|
No double bonds between C atoms | One or more double bonds between C atoms |
|
|
![]() Phospholipids
Phospholipids
Phospholipids are specialised lipids that are similar to triglycerides but one of the fatty acids is substituted for a phosphate group.
Phospholipids | Triglyceride |
A phospholipid has a hydrophobic part and a hydrophilic part.
Phospholipids are composed of:
- a hydrophilic head (attracts water) which consists of the glycerol and phosphate.
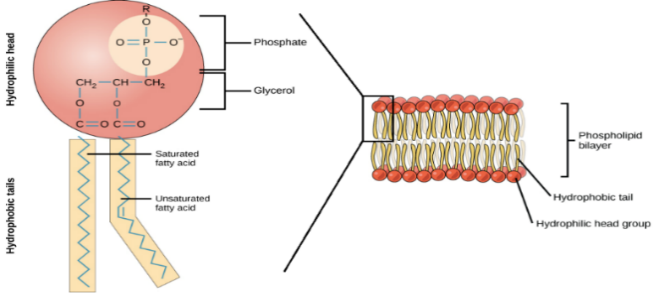 2 hydrophobic fatty acid tails (repel water). Remember these can be saturated or unsaturated and are insoluble in water because the fatty acids are non-polar.
2 hydrophobic fatty acid tails (repel water). Remember these can be saturated or unsaturated and are insoluble in water because the fatty acids are non-polar.
When multiple phospholipids come together in water, they form a bilayer. These phospholipid bilayers are what make up cell membranes (more on this later in Unit 2).
The structure of phospholipids is well suited to their function as forming cell membranes:
Structure of phospholipids | Function of phospholipids |
Hydrophilic heads and hydrophobic tail | Can form a double layer (bilayer) with heads facing out towards water on each side. |
Centre of the cell membrane bilayer is hydrophobic | Water-soluble substances can’t pass through easily (forms a barrier). |
![]() Test for lipids
Test for lipids
The test for lipids is known as the ethanol emulsion test;
- Using a completely dry clean test tube add ethanol to the sample to be tested.
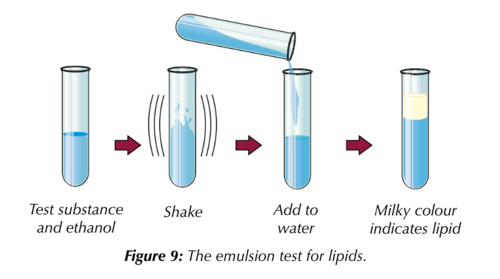 Shake thoroughly to dissolve any lipid in the sample.
Shake thoroughly to dissolve any lipid in the sample.- Add water to the sample and shake gently.
A positive result for the presence of lipids using this test is a milky white colour/emulsion.
This happens because the ethanol (alcohol) dissolves lipids.
(Note, you must not say a precipitate / cloudy emulsion).