12-01: Functional Groups and Linkages
Most of a living organism is water
The remaining amount of “stuff” in an organism falls into 4 main categories:
- Carbohydrates
- Lipids
- Proteins
- Nucleic Acids
Biochemistry
- Many cellular molecules are complex and can be made up of thousands of atoms
- These molecules are made by stringing together many smaller units → Single units are called monomers, multiple units are called polymers
- Anabolic reactions: build up substances
- Catabolic reactions: break down substance
- Both catabolic and anabolic reactions occurring in an organism: metabolism
Intramolecular bonds: bonds within a molecule
3 types of intramolecular bonds
- Covalent bonds
- 2 atoms share electrons (e-) → 2 non metals
- Ionic bonds
- 1 atom loses an e- and 1 gains an e-
- Polar covalent bonds
- Sharing the e- less fairly
Electronegativity (EN): the strength in which an atom attracts e- → how aggressive it is
The type of intramolecular bond is distinguished by a difference in electronegativity (∆EN)
- Covalent: ∆EN < 0.4 → share equally
- Polar covalent: ∆EN = 0.5-1.7
- Ionic: ∆EN > 1.7 → atom with greater EN takes e- from atom with lesser EN and both become charged
Polar Covalent Bonds
- One atom has a stronger hold on e- they share
- One end of the molecule gets slightly positively charged and one end gets slightly negatively charged
δ+ → one end gets + charge (weaker EN)
δ- → one end gets - charge (stronger EN)
This influences what will be attracted and how things will biologically interact
Water is polar → has polar covalent intramolecular bonds
- Because of Oxygen (O)’s EN, e- tend to spend more time near the O atom than near the Hydrogen (H) atoms
- The unequal sharing e- creates a slight difference in the charge between the ends/poles of the molecule
- the O end is δ- and the H ends are δ+
- Due to its polarity, water forms H bonds with itself
- Very weak bonds, but many of which come together and become very strong together
Water also forms bonds through….
- Cohesion: water molecules are attracted to other water molecules
- Adhesion: water is a polar molecule and thus attracts other polar molecules
Intermolectular forces: bonds between molecules
- ==London dispersion forces==: very weak attraction between molecules, even non polar ones. Increases with molecule size
- @@Dipole dipole attraction@@: attractive force between 2 polar molecules
- @@H bonding:@@ special kind of dipole dipole attraction; between 2 polar molecules with Hydrogen bonded to N, O, or F
Carbon - the element of life
- Backbone of nearly every biological molecule (except for water)
- Organic compound: compound that contains carbon-hydrogen bonds (may also contain other elements like O, N, etc) and is often found in organisms
- ^^C and H form a non polar bond so hydrocarbons are non polar^^
- Polarity can be achieved by adding other atoms called functional groups (FGs)
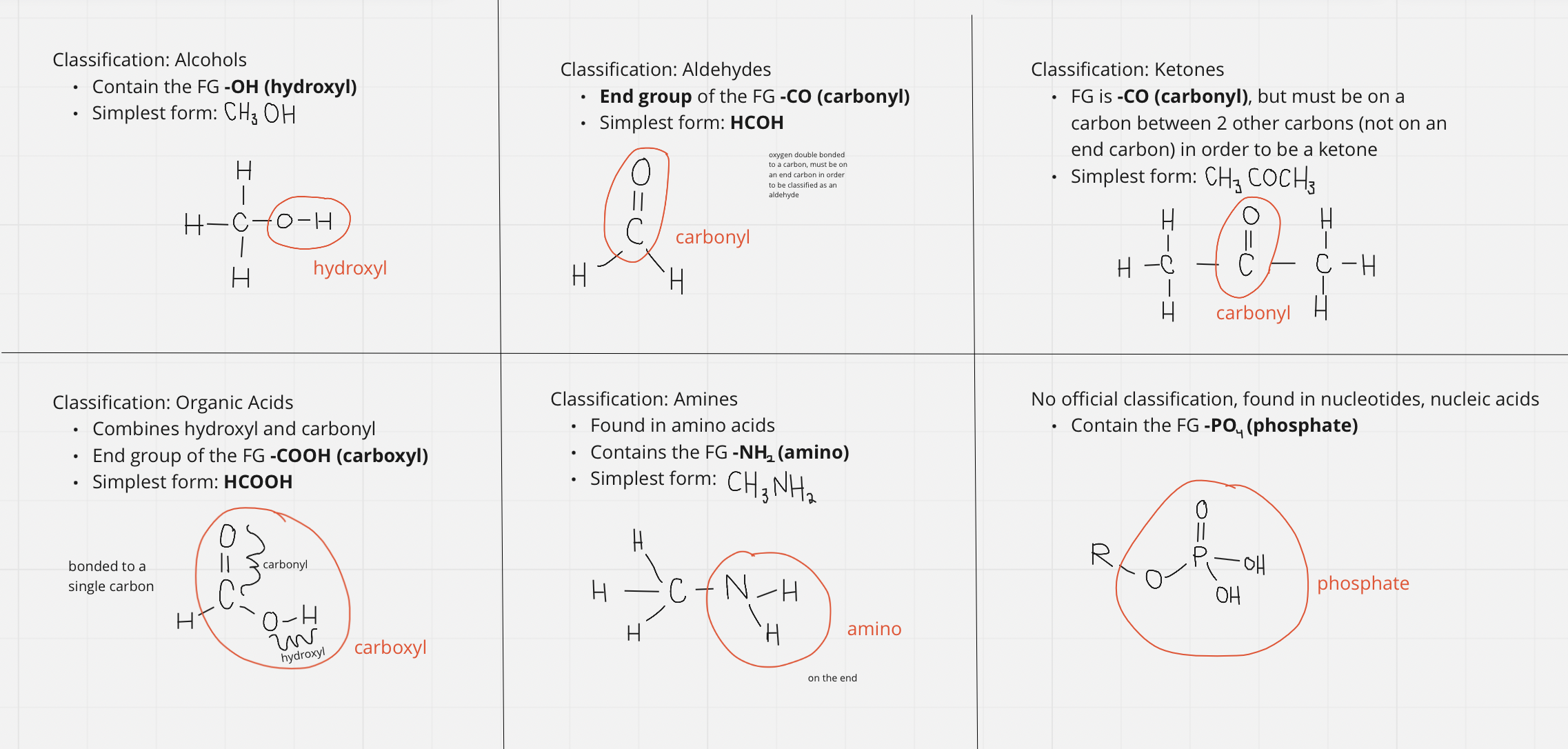
- Functional Groups -
- Molecules interact with each other at specific regions of their molecules (^^changes the dynamic of the molecule that allow it it interact differently^^)
- Used to classify molecule types
- After a reaction between 2 molecules’ functional groups, a linkage will be formed
- Monomers link to form polymers. This happens because of FGs reacting
Classifying FGs: if it has the FG, then it is classified that certain way
Types of Reactions
2 types:
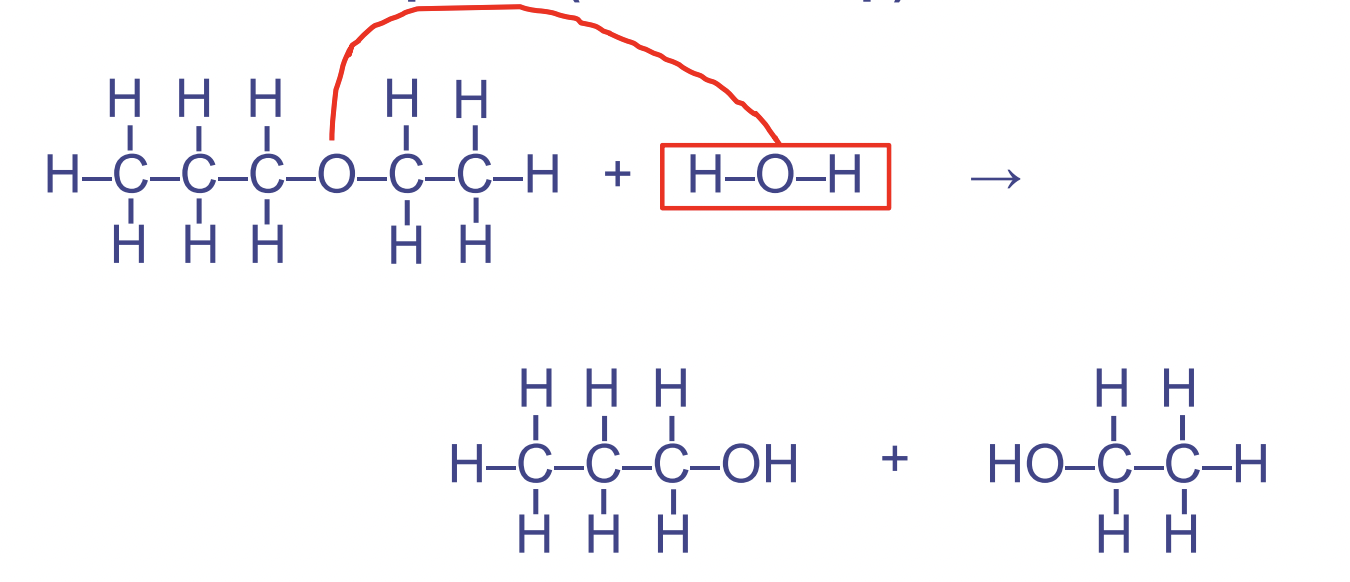
1. Hydrolysis
Rupture → use of water to rupture, break down
Catabolic
- Used to split larger molecules apart
Water is required and it is used up → it is a reactant
2. Dehydration synthesis (condensation)
Condenses smaller particles into larger ones
Anabolic
- Used to build up molecules
Water is released → it is a product
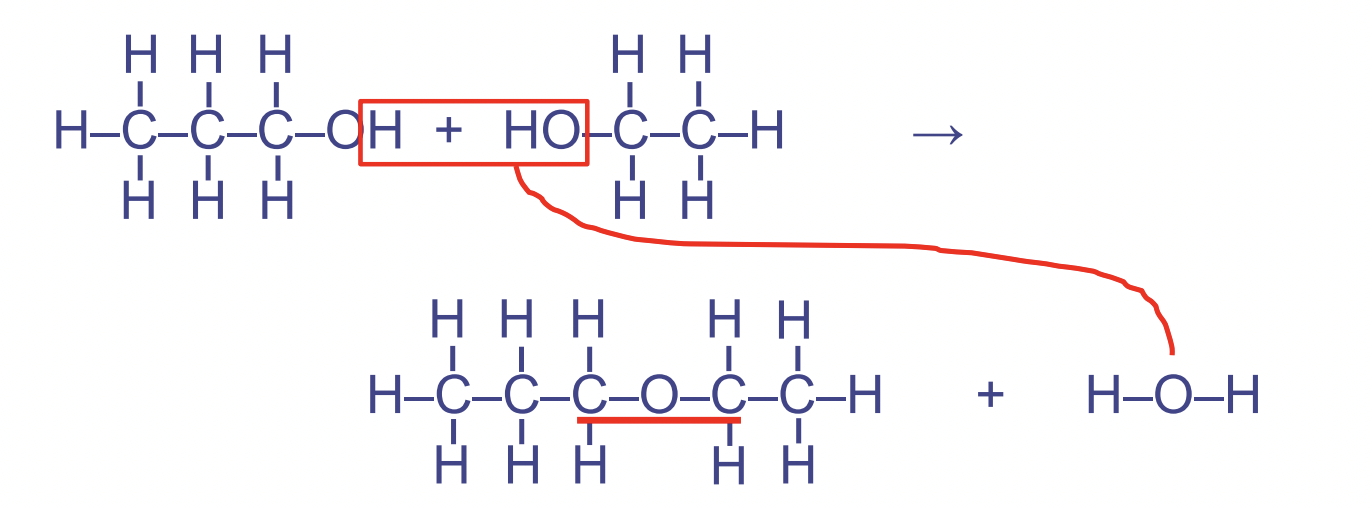
Linkages that form in dehydration synthesis reactions
Ether Linkages
(glycosidic link between sugars, when it occurs between sugar molecules)
Between %%2 hydroxyl groups%% (alcohols)
Used in carbohydrates
Forms the pattern COC
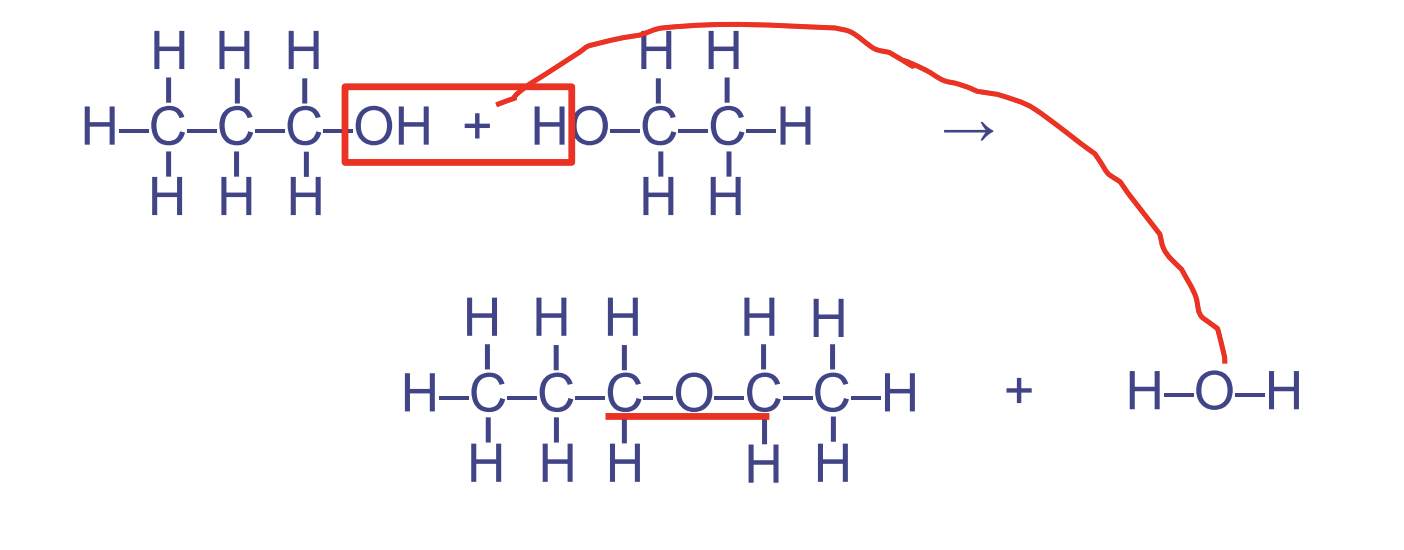
Ester Linkages
Between a %%hydroxyl and a carboxyl group%% (when they react)
Used in triglycerides
Forms the pattern OCO
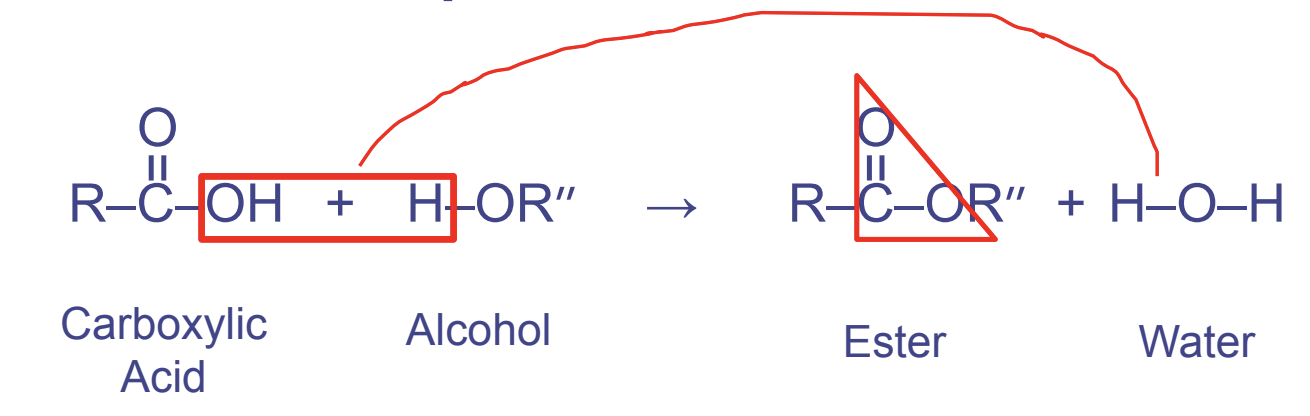
The double bond indicates that it is an ester linkage
Phosphate Ester Linkages
Between hydroxyl FGs (first) and phosphate FGs (second)
Used in phospholipids and nucleic acid, which instructs the cells, DNA and RNA
Forms the pattern OPO
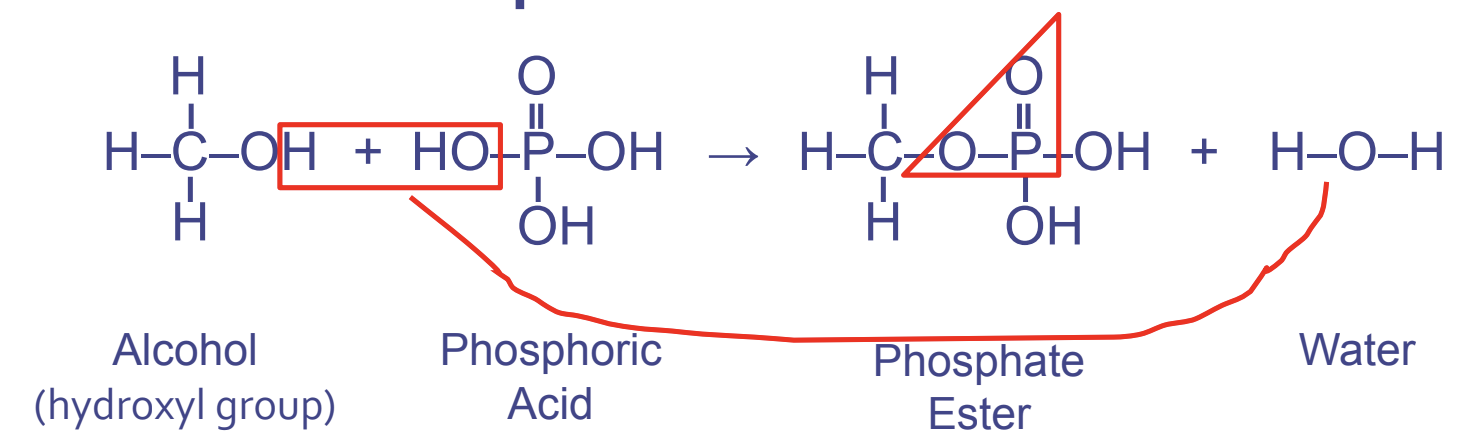
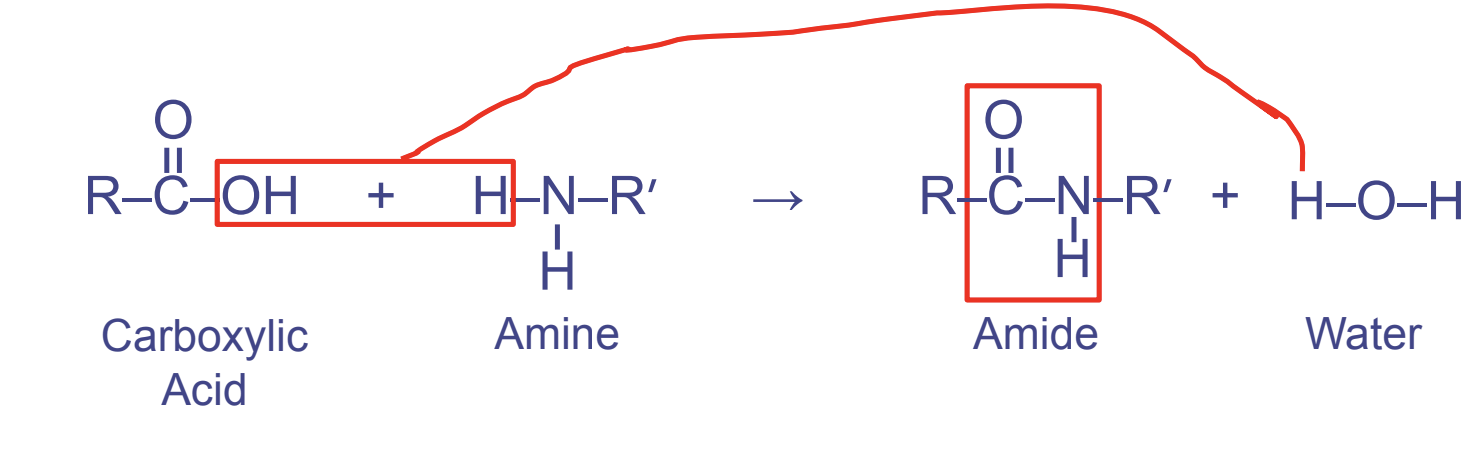
Peptide Linkages
Between carboxyl FGs (first) and amino FGs (second)
Links amino acids together
Forms the pattern OCNH