
amino acids and peptides
amino acids and peptides
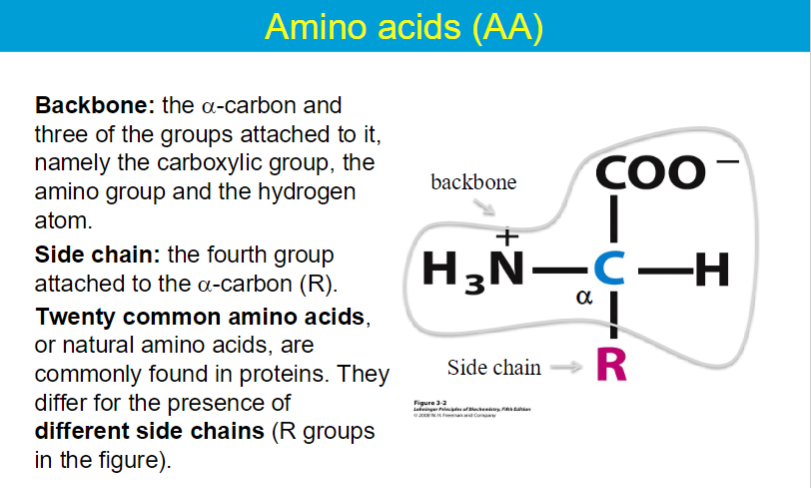
carboxyl group → acidic
amino group → basic
** all amino acids are chiral (except glycine)
D vs L amino acids??
To determine if an amino acid is L or D, you can look at the alpha carbon and follow the order from COOH to R to NH. If the order is counterclockwise, it's an L-amino acid, and if it's clockwise, it's a D-amino acid.
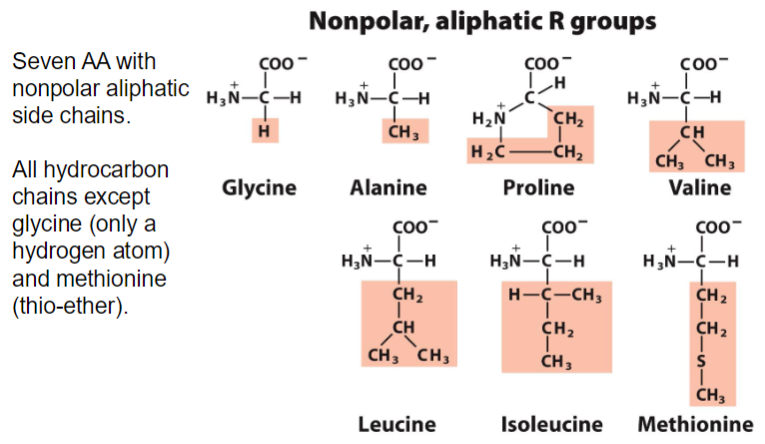
20 natural amino acids yippee
classified into 5 groups
nonpolar, aliphatic
aromatic
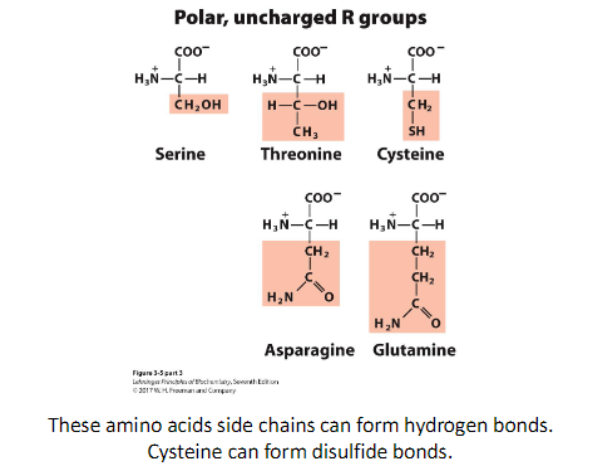
polar, uncharged
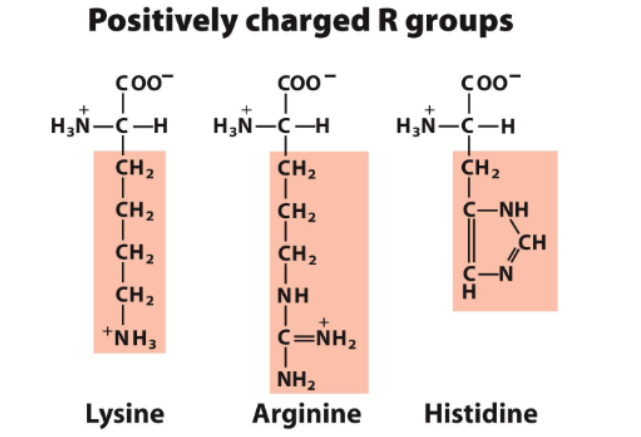
positively charged
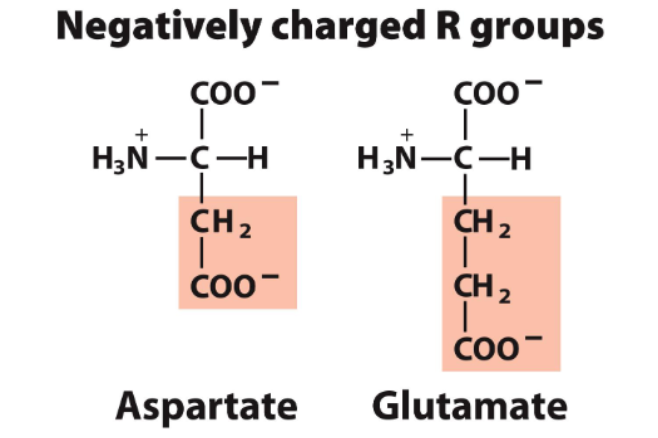
negatively charged
** memorize 20 amino acid names, 3 letter abv, and 1 letter abv
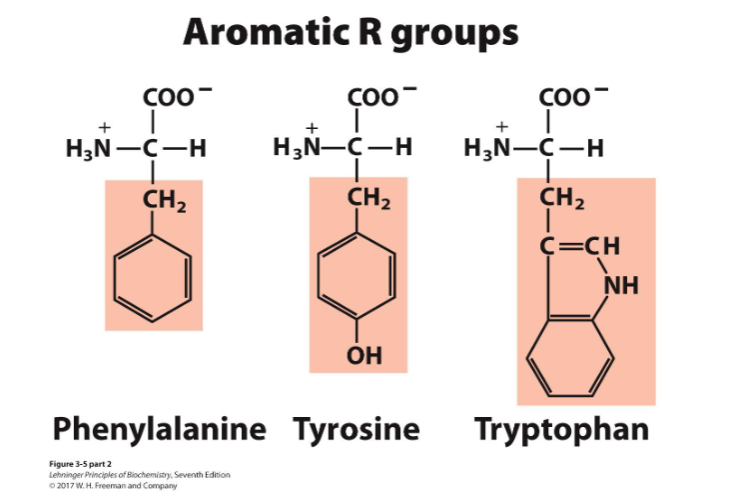
aromatic amino acids can absorb UV light
IONIZATION OF AMINO ACIDS
At a pH below its pKa, a molecule will be primarily protonated.
At a pH above its pKa, a molecule will be primarily deprotonated.
carboxylic acid (low pKA) → protonated at a low pH (lower than pKa)
ion form until pH is lower than pKa
COOH → COO- protonated → deprotonated
amino acid (high pKa) → deprotonated at high pH (higher than pKa
protonated until pH is higher than pKa
NH3+ → NH2 protonated → deprotonated
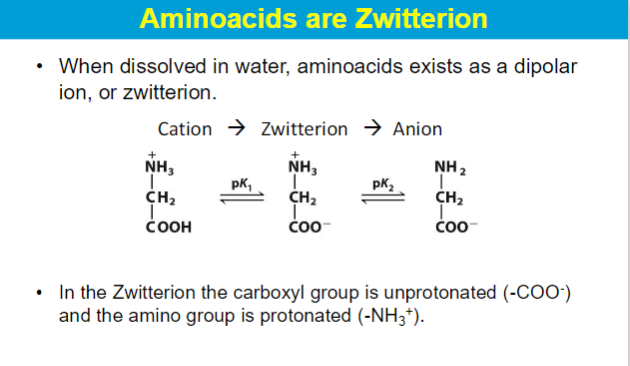
cation: low pH → carboxyl group protonated (/), amino group protonated (+)
water pH 7 → carboxyl group deprotonated (-), amino group protonated (+)
anion: high pH → carboxyl group deprotonated(-), amino group deprotonated (/)
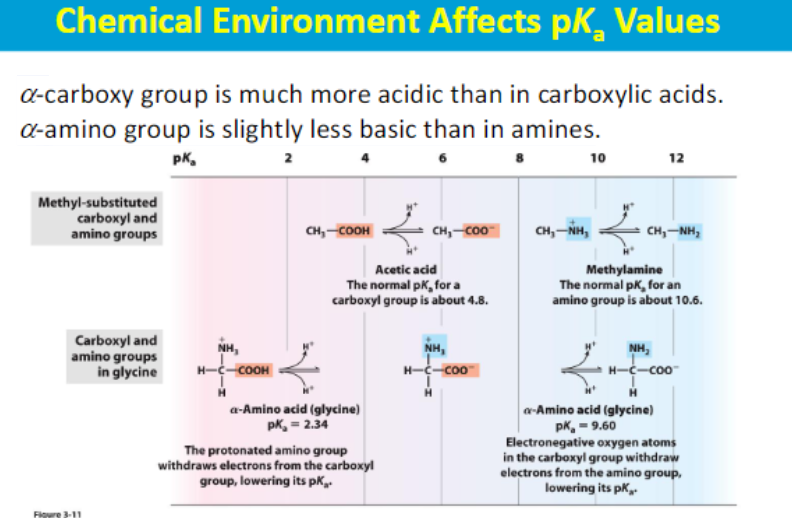
When an amino group (with a positive charge) is directly adjacent to a carboxyl group, it exerts a negative inductive effect, which helps stabilize the negative charge on the carboxylate ion formed after deprotonation, making the carboxylic acid more acidic.
Conversely, the nearby negatively charged carboxylate ion can slightly decrease the electron density on the amino group, making it less readily available to accept a proton and thus, slightly less basic compared to a typical amine ????
**Zwitterions predominate at pH values between the pKa values of the amino and carboxyl groups
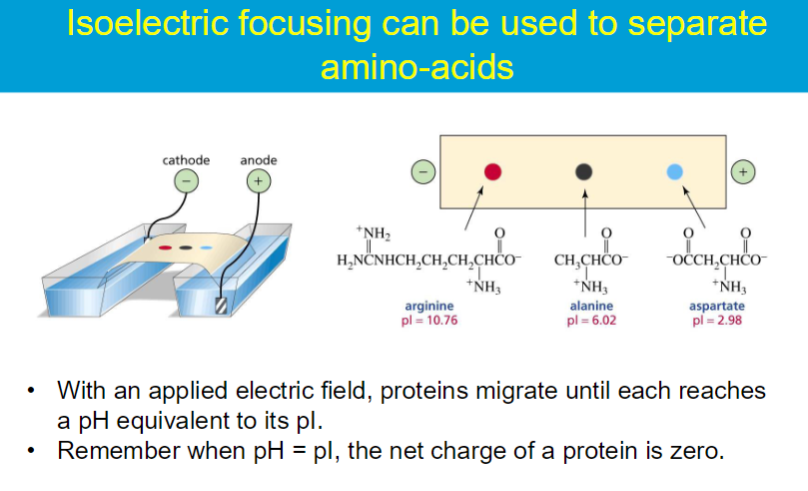
isoelectric point (pI) - the pH in which the amino acid has a net charge of 0
when AA is least soluble in water
AA does not migrate in electric field
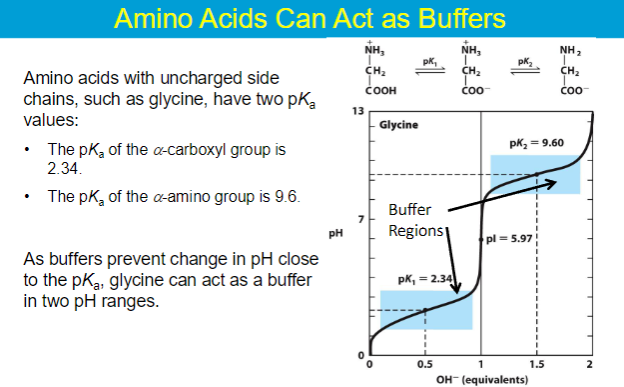
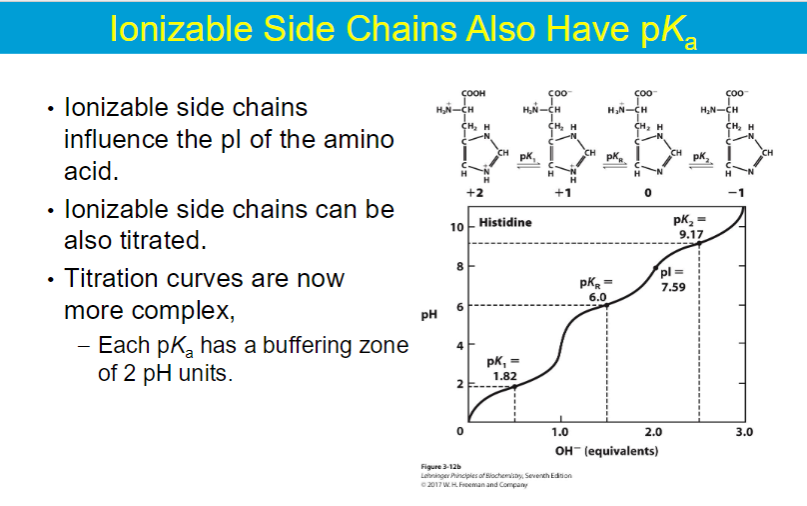
**in amino acids where the side chain is NON-IONIZABLE the following equation can be used
pI = (pKa1 + pKa2) / 2
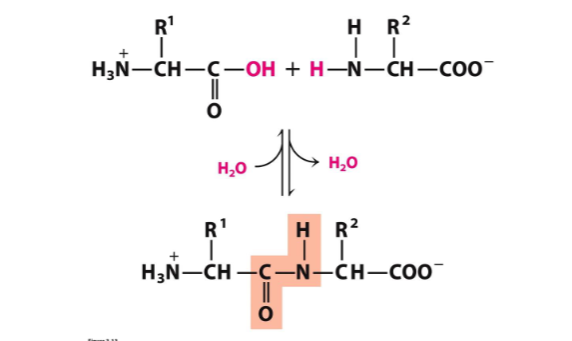
peptide bond formation
peptides have an amino (N) terminus and carboxyl (C) terminus
numbering and naming starts from the N terminus

dipeptide → 2 amino acids join
tripeptide → 3 amino acids
polypeptide → 10 or more amino acids
proteins → may contain 10,000 or more amino acids
amino acids and peptides

carboxyl group → acidic
amino group → basic
** all amino acids are chiral (except glycine)
D vs L amino acids??
To determine if an amino acid is L or D, you can look at the alpha carbon and follow the order from COOH to R to NH. If the order is counterclockwise, it's an L-amino acid, and if it's clockwise, it's a D-amino acid.
20 natural amino acids yippee
classified into 5 groups
nonpolar, aliphatic
aromatic
polar, uncharged
positively charged
negatively charged
** memorize 20 amino acid names, 3 letter abv, and 1 letter abv


aromatic amino acids can absorb UV light



IONIZATION OF AMINO ACIDS
At a pH below its pKa, a molecule will be primarily protonated.
At a pH above its pKa, a molecule will be primarily deprotonated.
carboxylic acid (low pKA) → protonated at a low pH (lower than pKa)
ion form until pH is lower than pKa
COOH → COO- protonated → deprotonated
amino acid (high pKa) → deprotonated at high pH (higher than pKa
protonated until pH is higher than pKa
NH3+ → NH2 protonated → deprotonated

cation: low pH → carboxyl group protonated (/), amino group protonated (+)
water pH 7 → carboxyl group deprotonated (-), amino group protonated (+)
anion: high pH → carboxyl group deprotonated(-), amino group deprotonated (/)


When an amino group (with a positive charge) is directly adjacent to a carboxyl group, it exerts a negative inductive effect, which helps stabilize the negative charge on the carboxylate ion formed after deprotonation, making the carboxylic acid more acidic.
Conversely, the nearby negatively charged carboxylate ion can slightly decrease the electron density on the amino group, making it less readily available to accept a proton and thus, slightly less basic compared to a typical amine ????
**Zwitterions predominate at pH values between the pKa values of the amino and carboxyl groups
isoelectric point (pI) - the pH in which the amino acid has a net charge of 0
when AA is least soluble in water
AA does not migrate in electric field
**in amino acids where the side chain is NON-IONIZABLE the following equation can be used
pI = (pKa1 + pKa2) / 2


peptide bond formation

peptides have an amino (N) terminus and carboxyl (C) terminus
numbering and naming starts from the N terminus

dipeptide → 2 amino acids join
tripeptide → 3 amino acids
polypeptide → 10 or more amino acids
proteins → may contain 10,000 or more amino acids