Organic Chemistry
Key Vocabulary:
Fractional distillation: The process by which a mixture of miscible liquids is separated (by heating with repeating evaporation and condensation) due to differences in their b.p.
Empirical formula shows the simplest whole number ratio of the atoms of each element in the molecule
Substitution: a type of reaction in which one atom is replaced by another
Polymer: long chain molecule
Monomer: small molecule used to form a polymer
Viscous: Thick sticky liquid (like honey)
Volatile: a liquid that readily turns into a gas
flammable: a substance that readily ignites
Introduction:
Organic chemistry studies carbon-containing compounds, except for carbon oxides (CO₂ and CO), which are inorganic.
These compounds are often derived from living organisms.
Carbon forms strong covalent bonds and can form 4 bonds (single, double, or triple) due to having 4 electrons in its outer shell.
Fossil Fuels:
Fossil fuels include crude oil, natural gas, and coal.
They were formed from the remains of organisms that lived millions of years ago.
Fossil fuels are finite resources and will eventually run out.
Crude oil:
Crude oil is a complex mixture of hydrocarbons, mainly.
Hydrocarbons are compounds that contain hydrogen and carbon only
Cruel oil is not directly used as a fuel because:
Viscous and does not burn easily.
A complex mixture of molecules with varying chain lengths and properties, needing separation first.
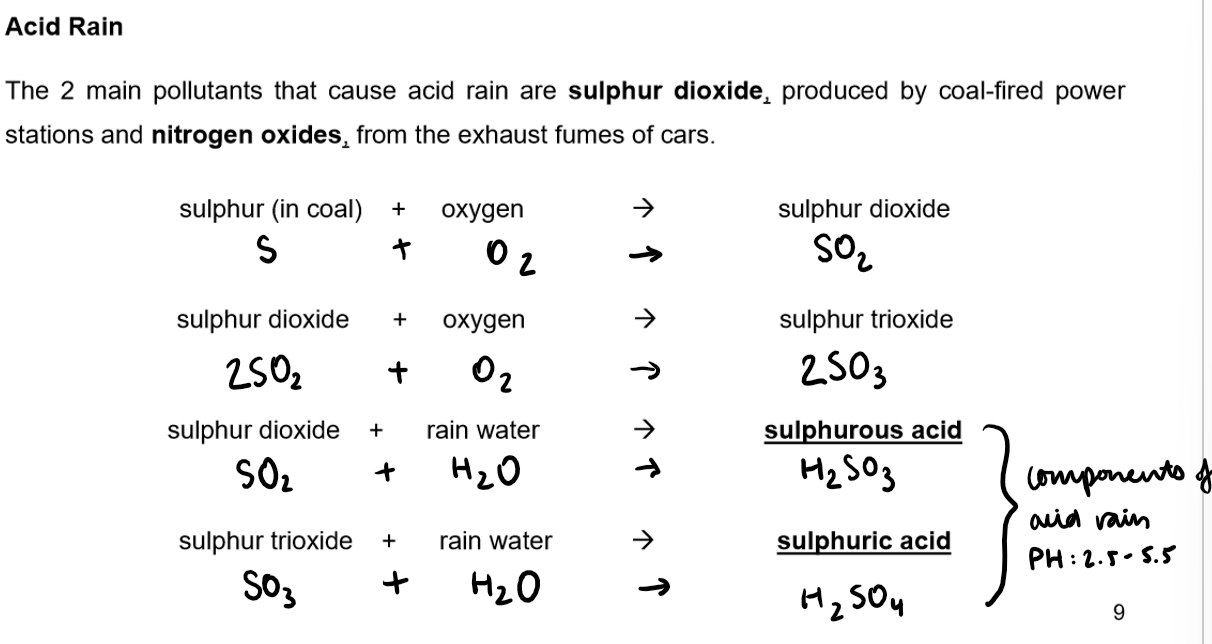
Burning fossil fuels can cause pollution, such as sulphur dioxide forming acid rain.
Industrial separation of crude oil into fractions by fractional distillation:
Crude oil is separated into fractions using fractional distillation in a column.
Fractions are mixtures of hydrocarbons with similar carbon numbers and boiling points.
Fractional distillation separates liquids based on their boiling points
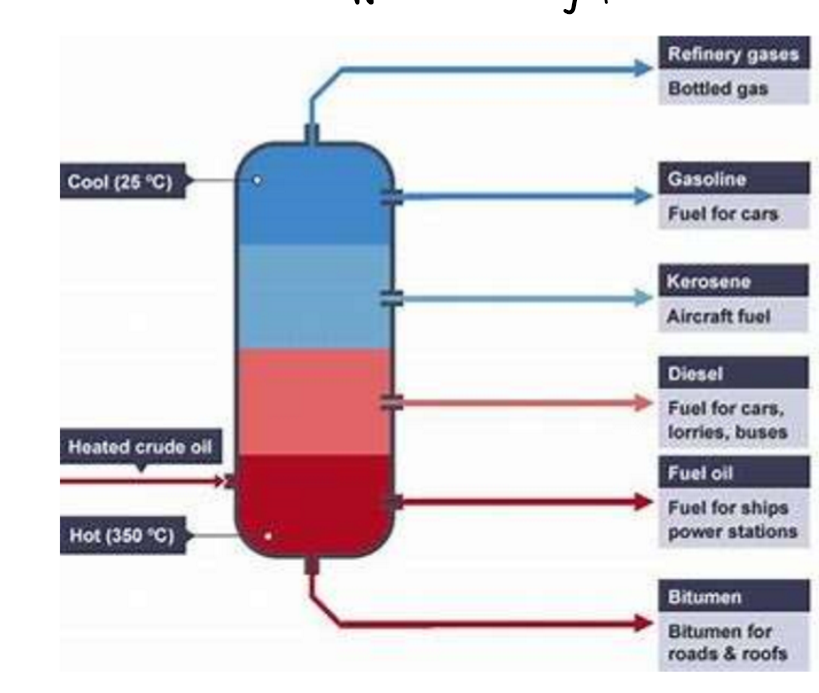
Process of heating crude oil:
Crude oil is heated, causing compounds to evaporate and enter the lower part of the fractionating column.
The column is hottest at the base and cooler at the top.
Vapours rise until they condense at heights matching their boiling points.
Smaller molecules have lower boiling points, so they rise higher before condensing.
Fractions are collected at different heights in the column.
Evaporation and condensation occur during the process.
Crude oil separates into fractions due to differences in carbon atom numbers and boiling points.
Fractional distillation produces more long-chain hydrocarbons (commercially useless) and fewer short-chain hydrocarbons (commercially useful).
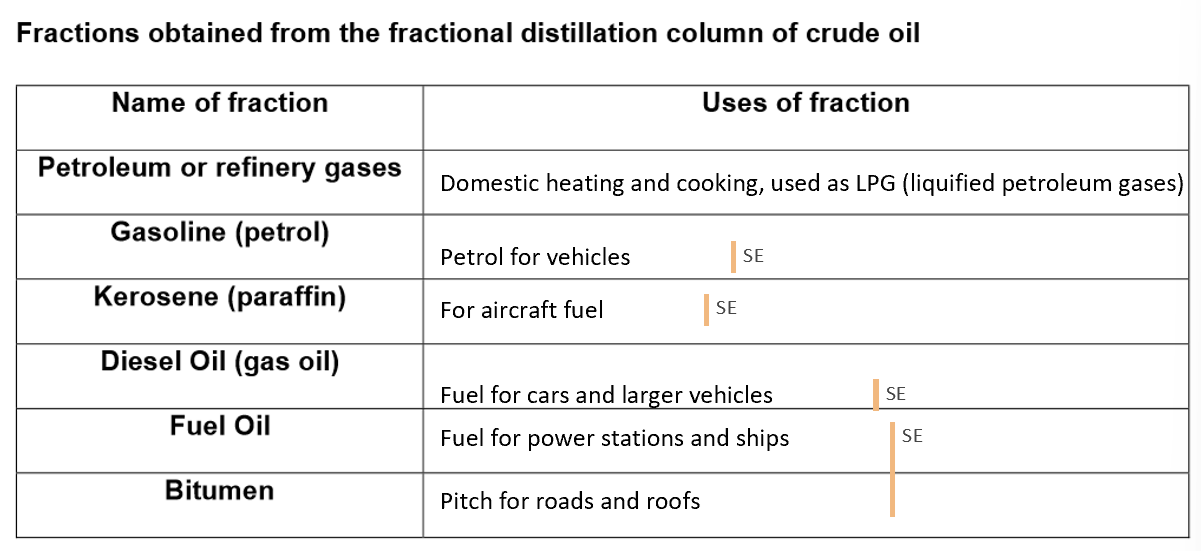
As the molecules get larger, the fractions will get more viscous, less flammable, less volatile and darker in colour, burn with smokier flames and their boiling points will increase.
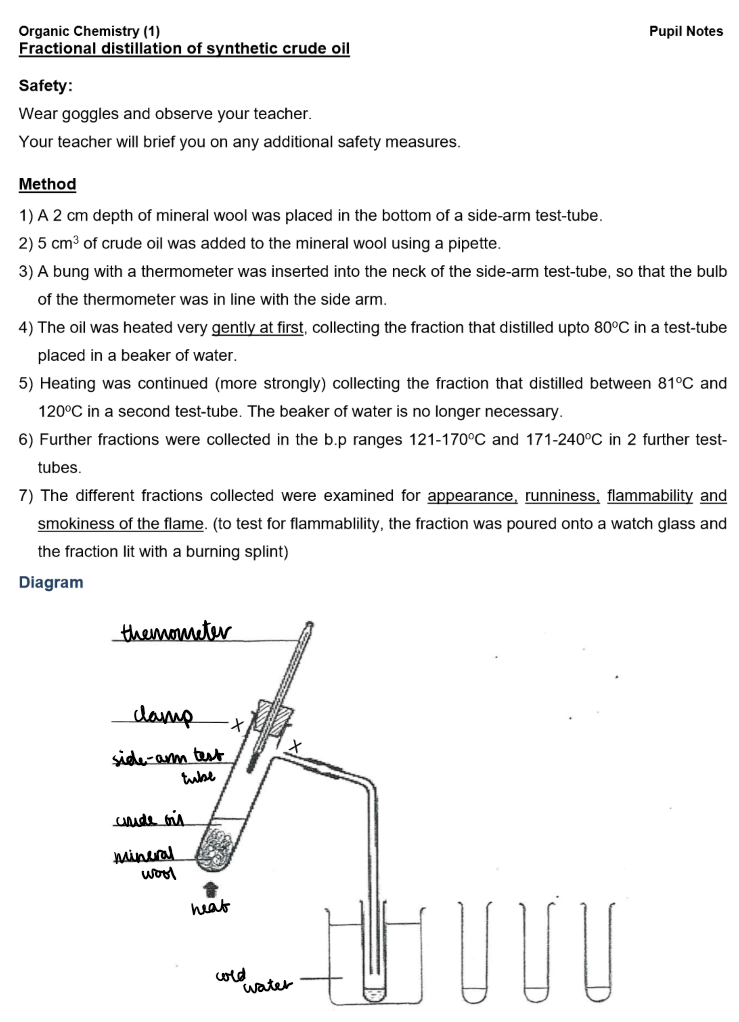
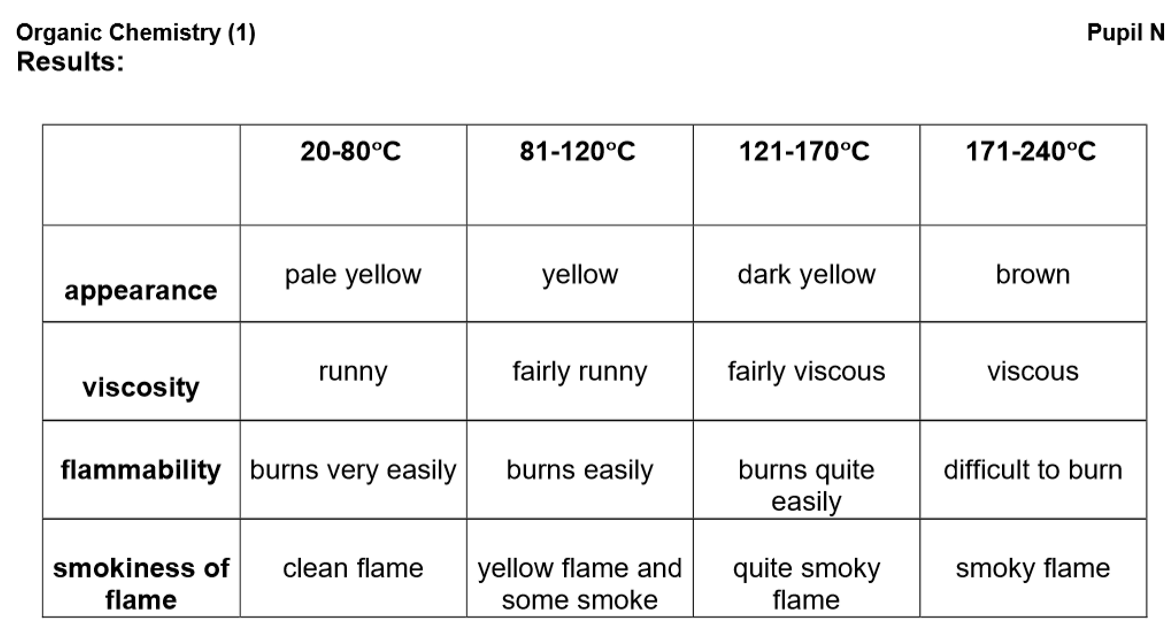
Conclusion:
Lower boiling point fractions have paler colours, lower viscosity, greater flammability, and higher volatility.
These fractions have fewer carbon atoms, meaning they have weaker intermolecular forces that require less energy to overcome

ORGANIC REACTIONS: STATE SYMBOLS ARE NOT NEEDED
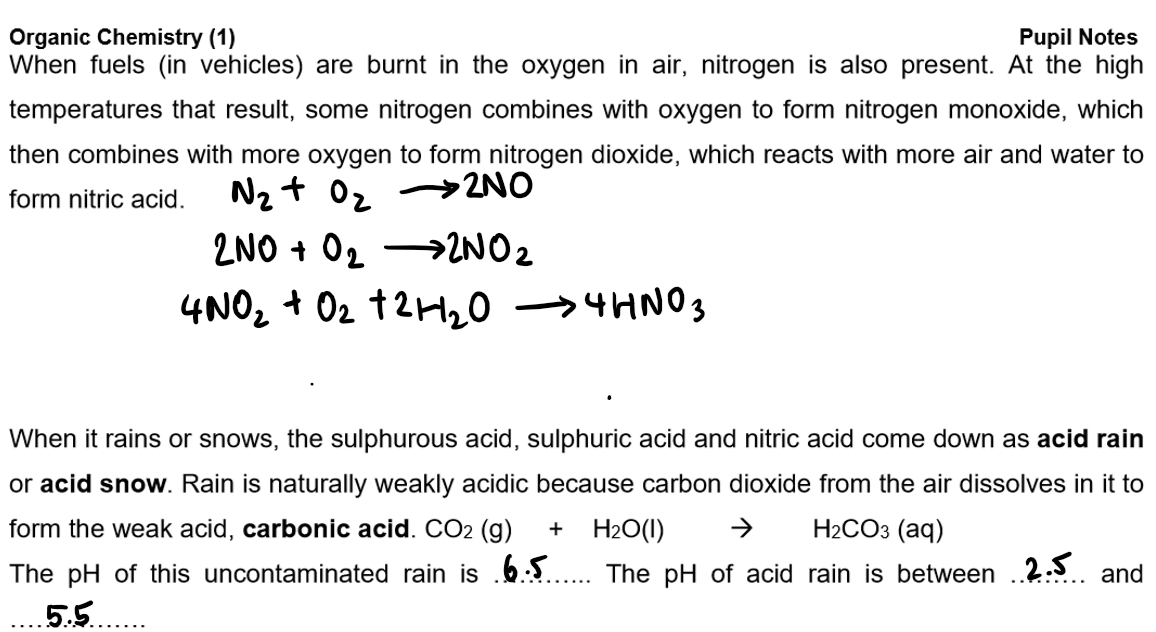
Burning of fossil fuels:
they are used as fuels because they give out plenty of heat energy when they burn in a plentiful supply of oxygen, producing carbon dioxide and water vapour (complete combustion)

If there is not an adequate supply of oxygen, there is incomplete combustion. This leads to the formation of carbon monoxide or carbon (soot) instead of carbon dioxide
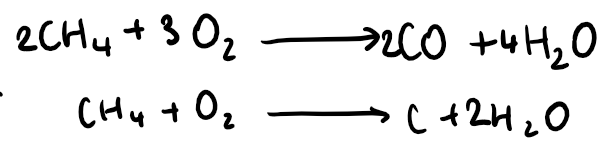
CO is colourless, odourless, and highly toxic. It is poisonous because haemoglobin prefers to bind with it over oxygen, leading to oxygen displacement in the body, which can be fatal.
Problems with Burning Fossil Fuels:
Fossil fuels contain sulphides that, when burned, form sulphur dioxide.
Sulphur dioxide contributes to acid rain.
Power stations, burning large amounts of coal, are significant contributors to this issue

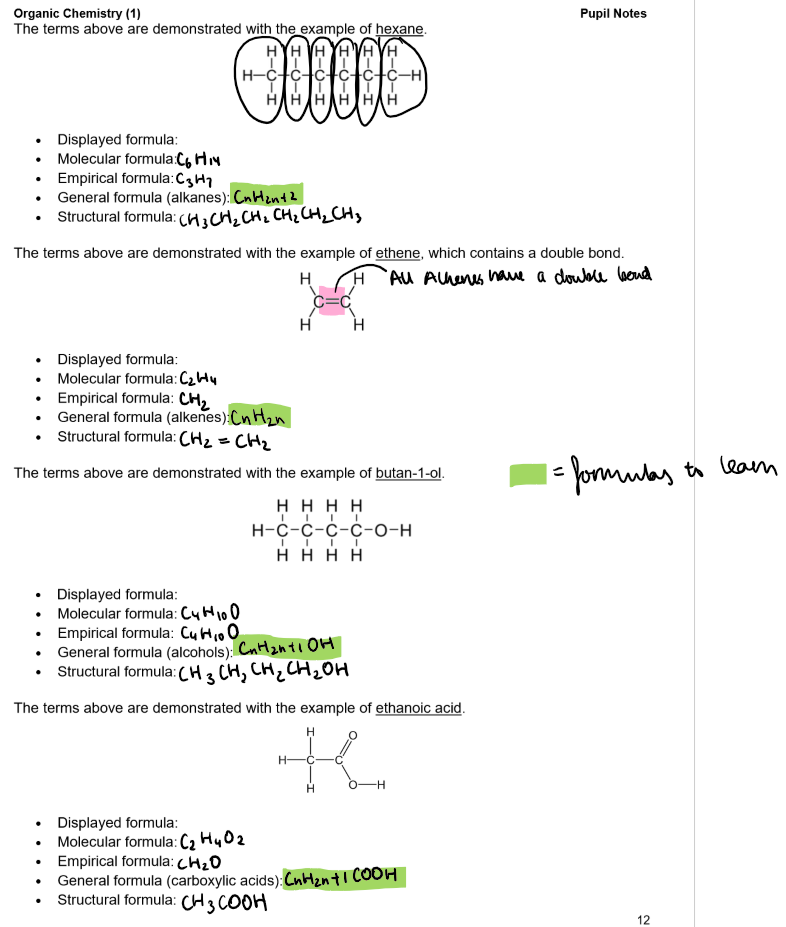
Types of Formulas for Organic Molecules:
Molecular Formula:
Shows the actual number of atoms of each element in the molecule.
E.g., the molecular formula of butane is C₄H₁₀.
Empirical Formula:
Shows the simplest whole number ratio of the atoms of each element.
E.g., the empirical formula of butane is C₂H₅.
General Formula:
Represents any member of a homologous series.
E.g., the general formula for alkanes is CₙH₂ₙ₊₂ (where n is an integer).
Structural Formula:
Shows how the atoms are bonded in the molecule.
E.g., the structural formula of butane is CH₃CH₂CH₂CH₃.
Displayed Formula:
A type of structural formula that shows all the bonds present in the molecule.
E.g., the displayed formula of butane is: CH₃-CH₂-CH₂-CH₃.
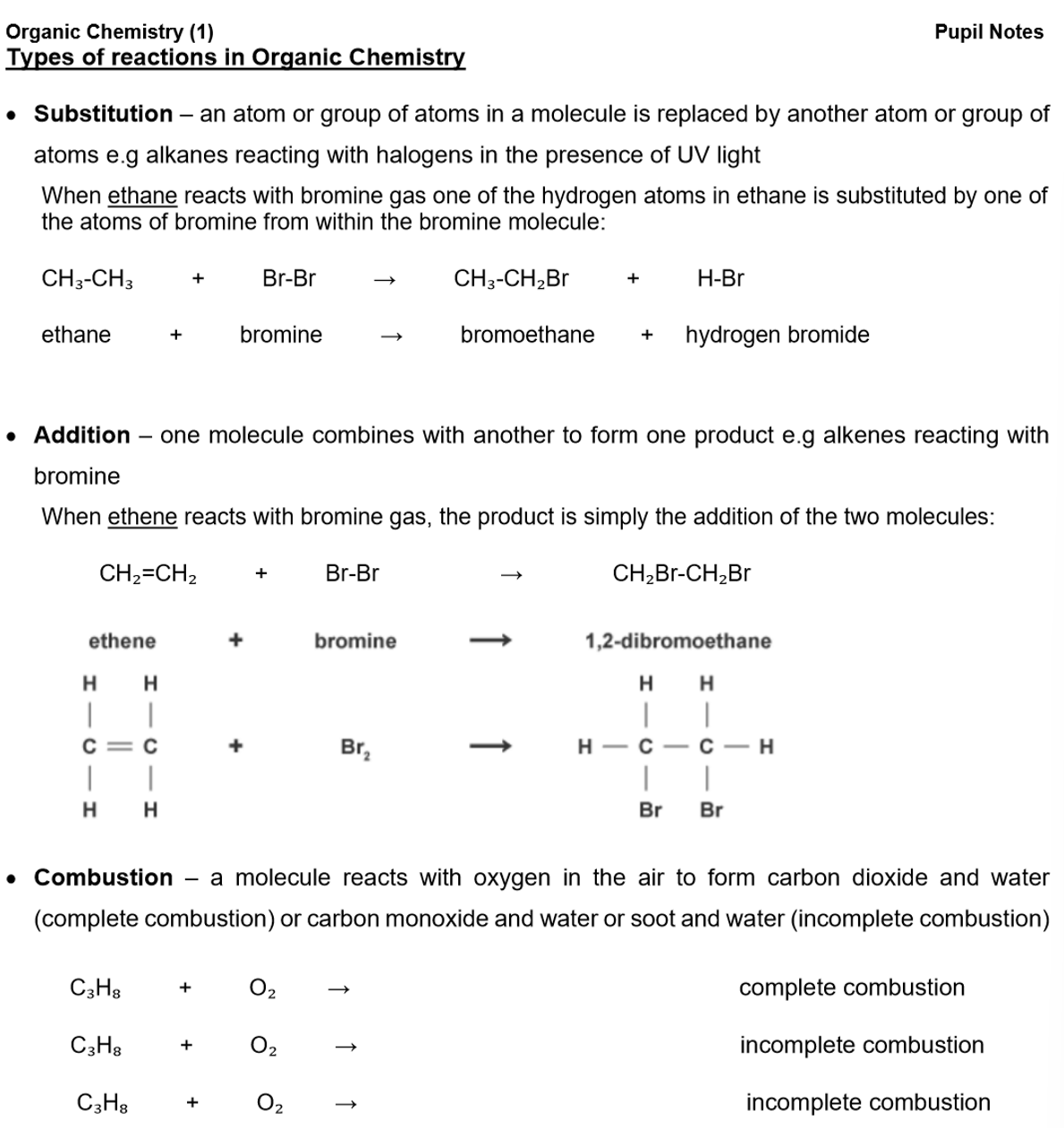
Alkanes:
Homologous Series: A group of compounds with common features.
Successive members differ by a CH₂ group.
Share the same general formula but have different carbon chain lengths.
Have the same functional group and similar chemical properties.
Show gradually changing physical properties (e.g., melting points, boiling points, viscosity).
Alkanes:
General Formula: CₙH₂ₙ₊₂.
Hydrocarbons: Contain only carbon and hydrogen atoms.
Saturated Compounds: Contain the maximum possible number of hydrogen atoms and cannot take on more.
Alkanes undergo substitution
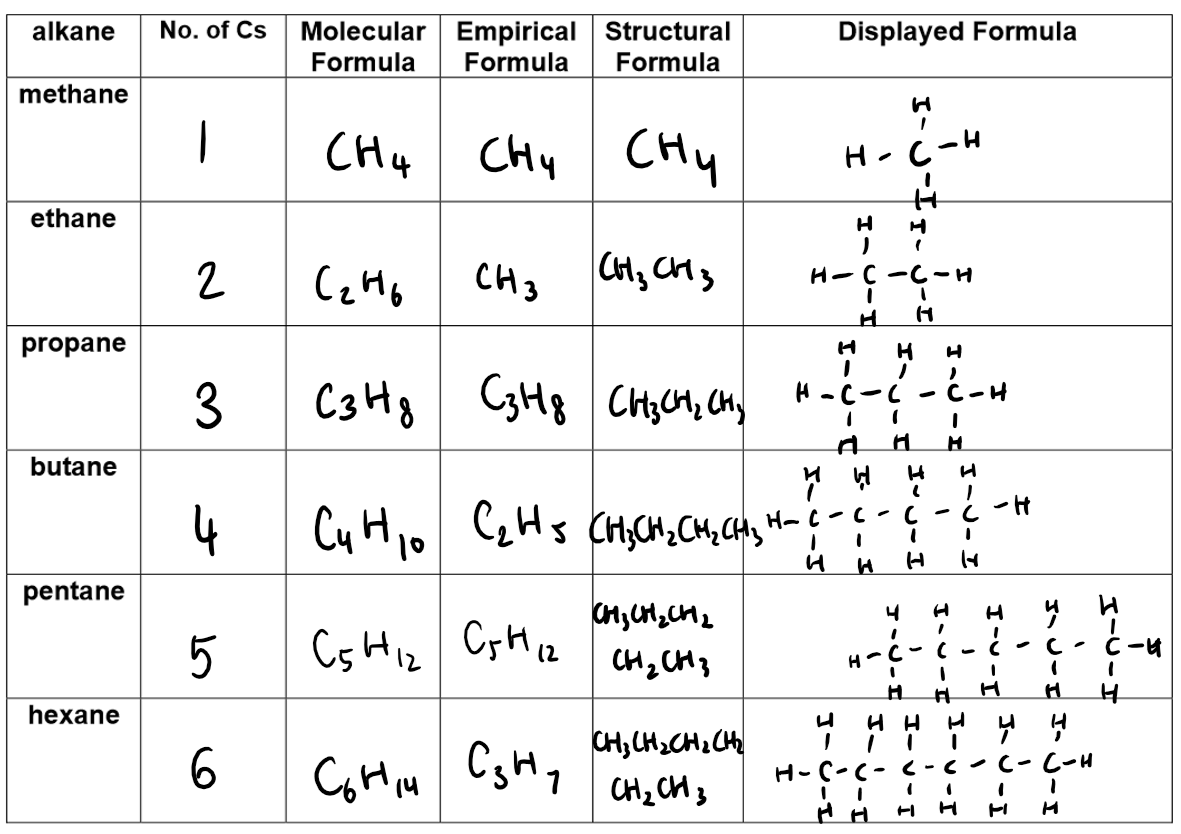
As the size of alkane molecules increases (more carbon atoms, longer chains), the following changes occur due to stronger intermolecular forces:
Boiling Point: Increases because stronger intermolecular forces require more energy to separate the molecules.
Viscosity: Increases as the liquid flows less easily due to stronger forces holding molecules together.
Volatility: Decreases as larger molecules evaporate more slowly due to stronger attractions.
Flammability: Decreases as larger molecules are less volatile, burn less easily, and produce smokier flames due to higher carbon content
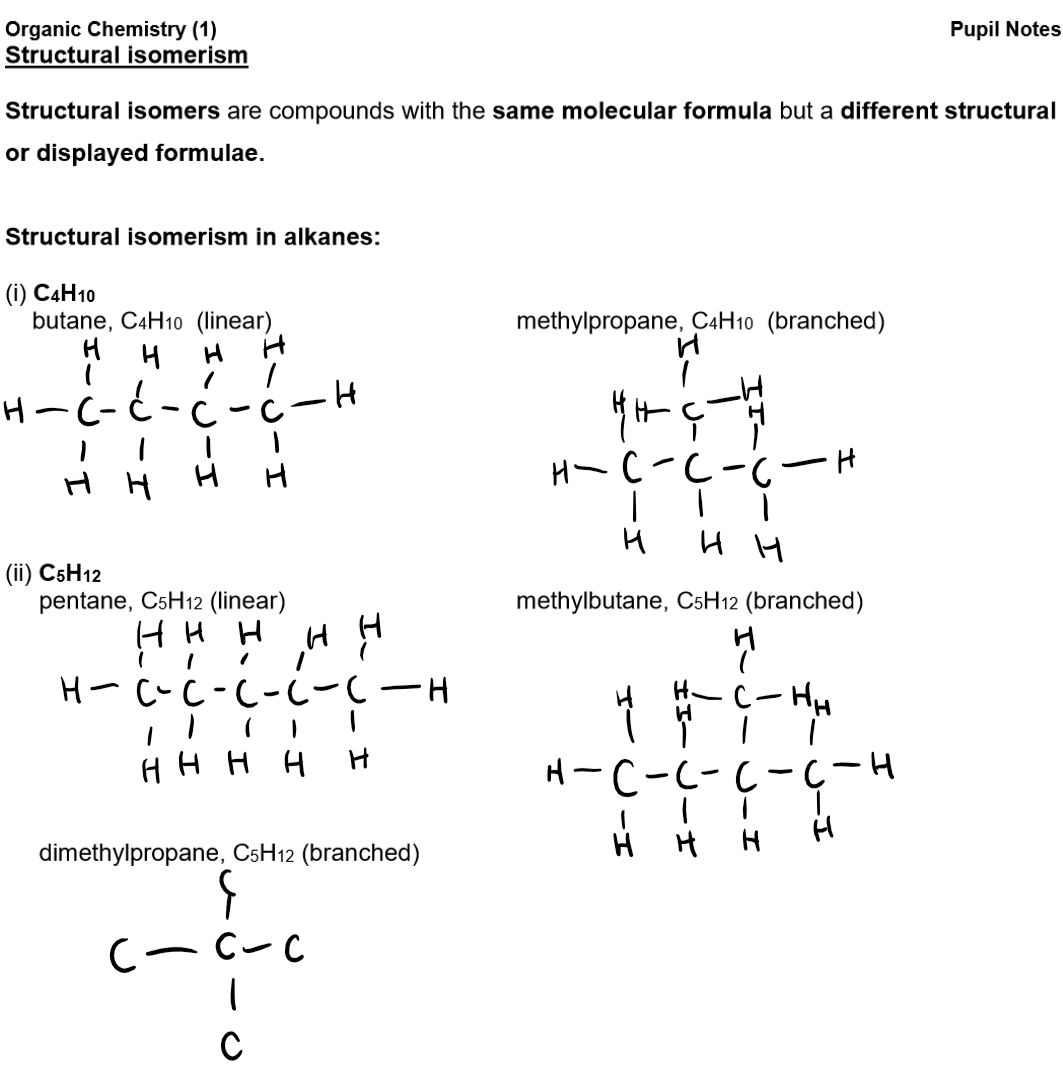
Physical Differences Between Isomers:
Weaker Intermolecular Forces: Branched-chain isomers have weaker attractions than straight chains due to reduced packing.
Reduced Surface Area: Branched molecules have less surface contact.
Lower Boiling Point: Weaker forces mean less energy is needed to separate molecules, so boiling points decrease with increased branching.
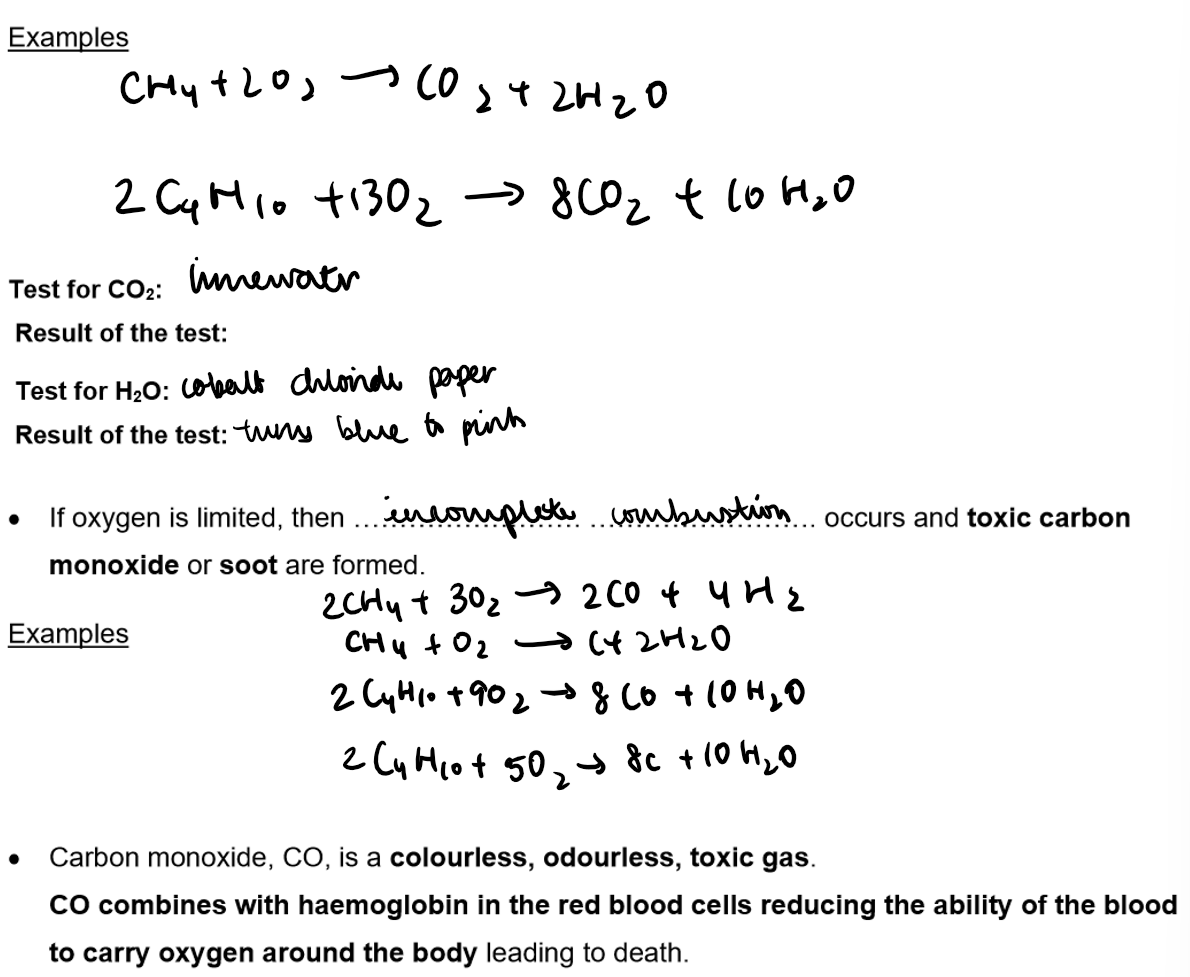
Reaction of Alkanes - Combustion:
Definition: Combustion is a redox reaction where fuels burn in oxygen, releasing heat energy.
Complete Combustion: Alkanes burn fully in excess air or oxygen, producing carbon dioxide and water.
Importance: Hydrocarbons, especially alkanes, are effective fuels due to their high energy output during combustion.
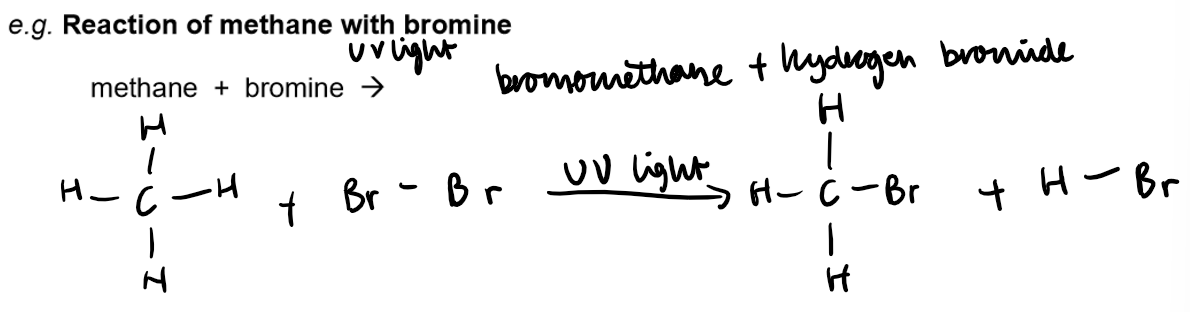
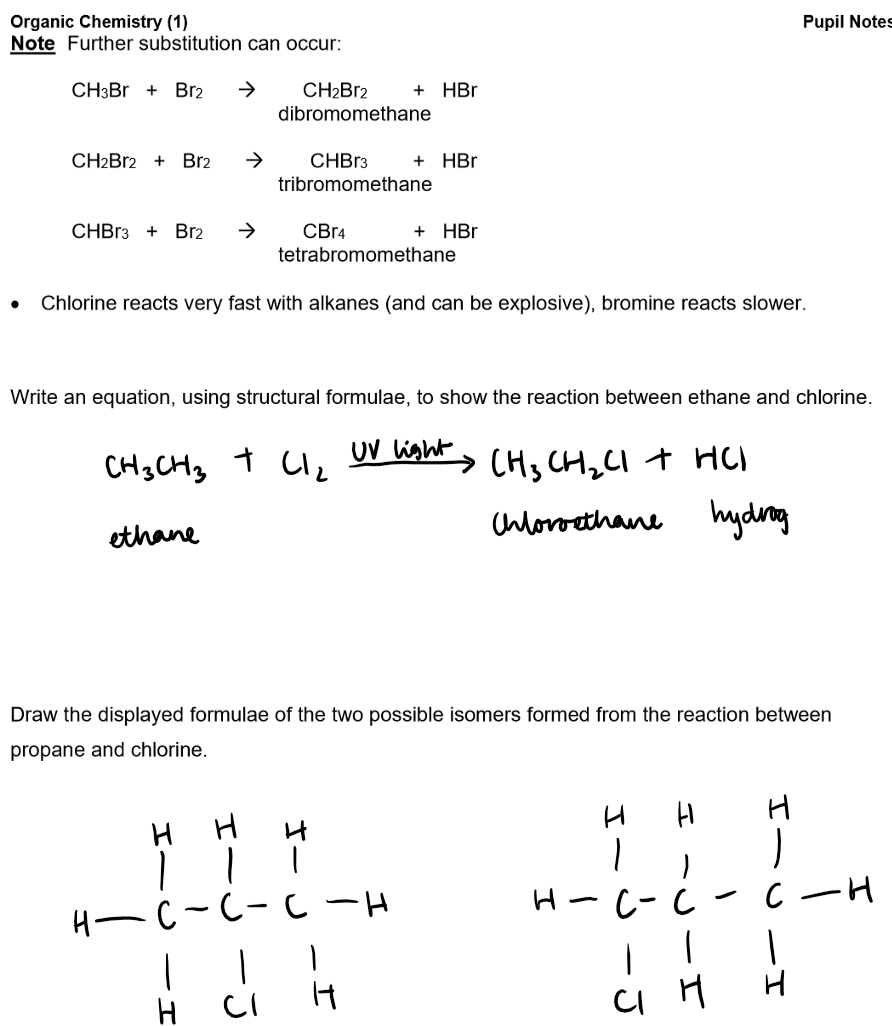
Substitution Reaction (Halogenation):
Reaction with Halogens: Alkanes react with halogens in the presence of UV light.
Definition: A substitution reaction replaces one or more hydrogen atoms in the alkane with halogen atoms.
General Principle: In substitution, an atom or group of atoms in a molecule is replaced by another atom or group
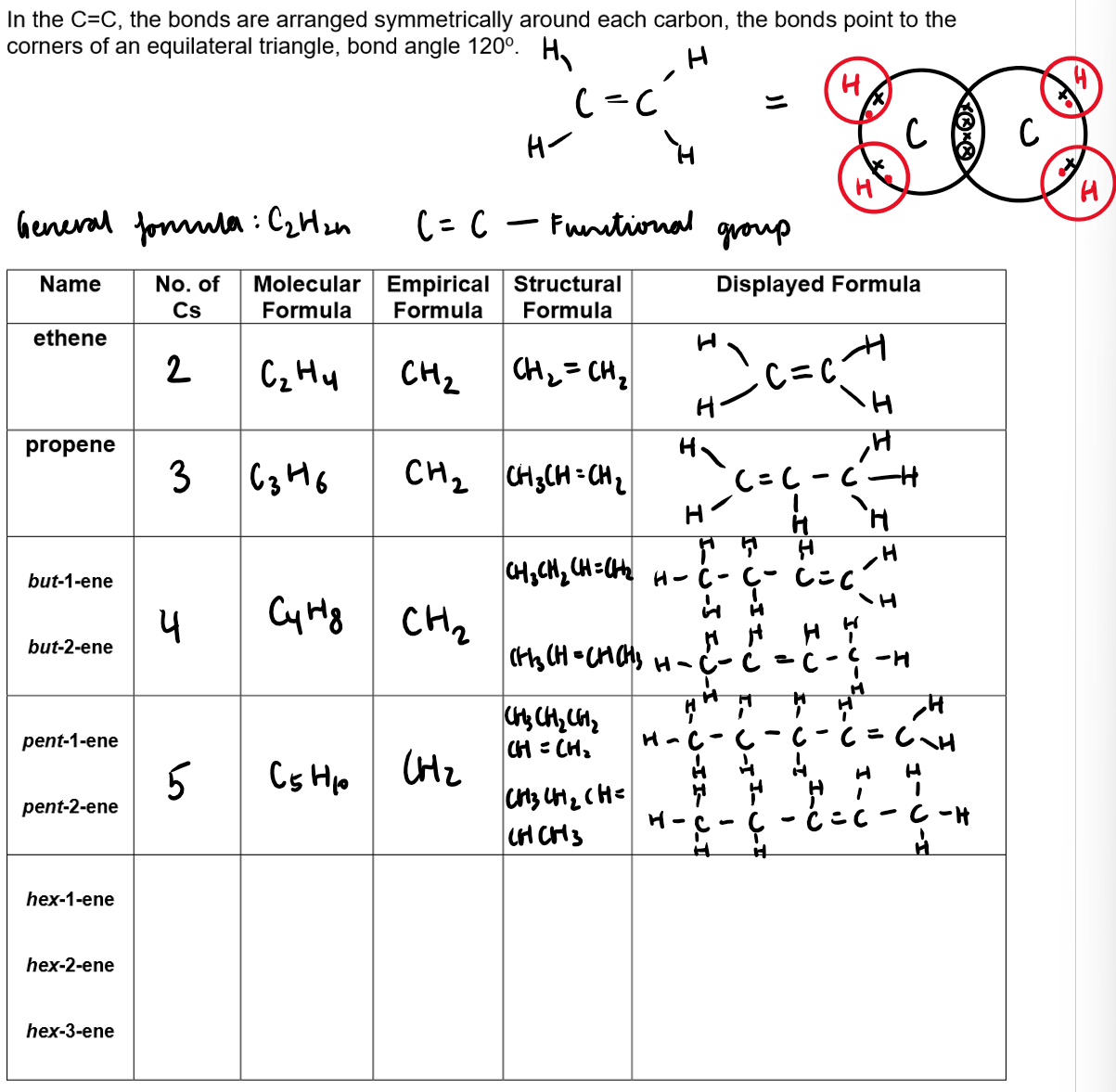
Alkenes:
The general formula of alkenes is CnH2n
Alkenes are hydrocarbon as they contain atoms of hydrogen and carbon only.
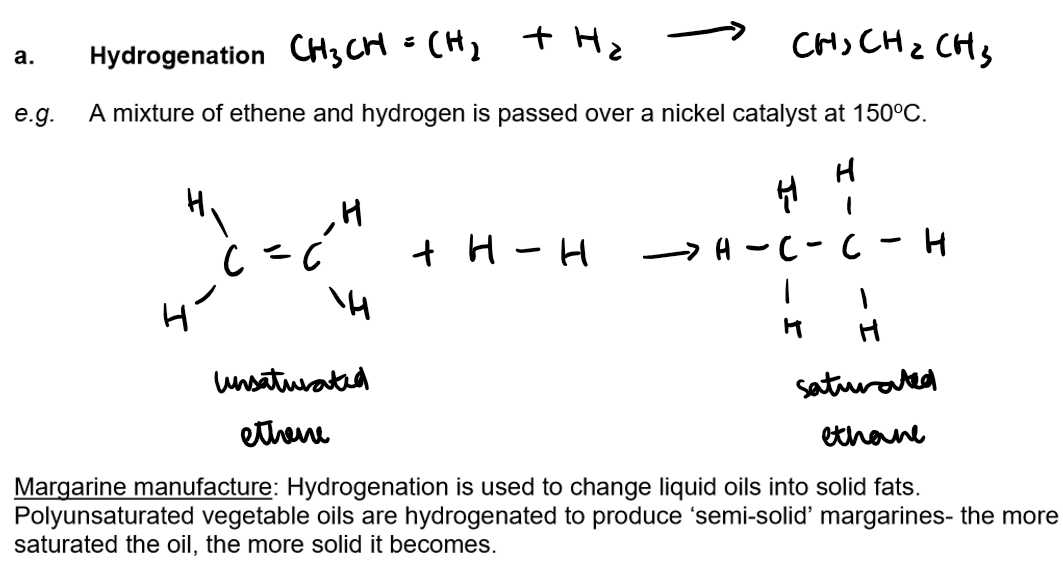
Alkenes undergo an addition reaction
Alkenes are unsaturated compounds:
They contain one double covalent bond between carbon atoms
The c=c double bond as the functional group can break and add on more atoms.
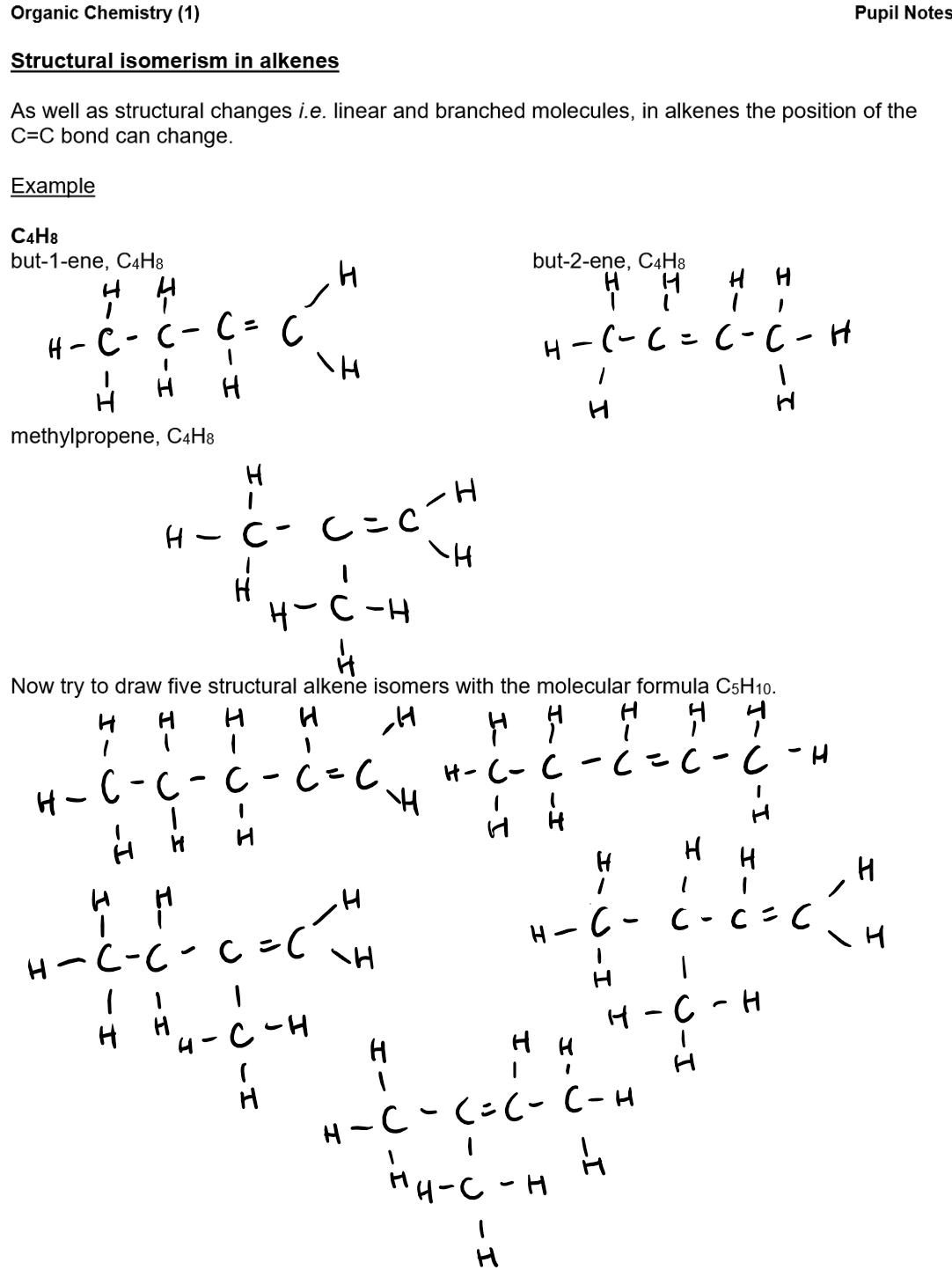
Reaction of alkenes:
Combustion:
Alkenes undergo complete combustion when burnt in plentiful air or oxygen, producing carbon dioxide and water.
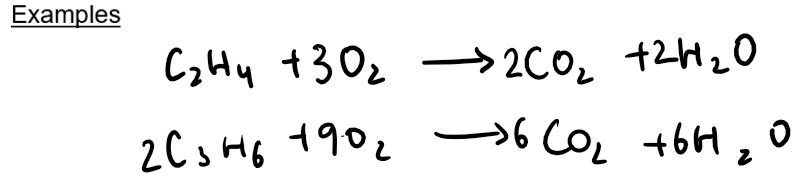
If oxygen is limited, then incomplete combustion occurs and toxic carbon monoxide or soot are formed
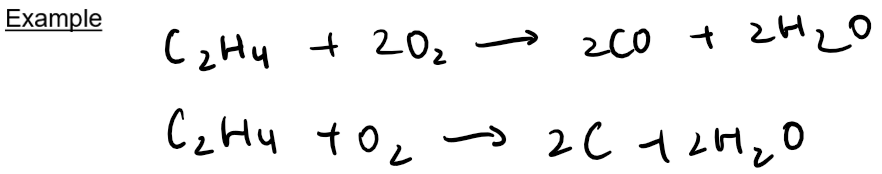
Additional reactions:
In an addition reaction, 2 molecules react to from one molecule
One of the carbon-carbon double bond breaks and a small molecule is added across the c-c bond
Alkenes undergo addition reactions and become saturated e.g. they are converted to alkanes

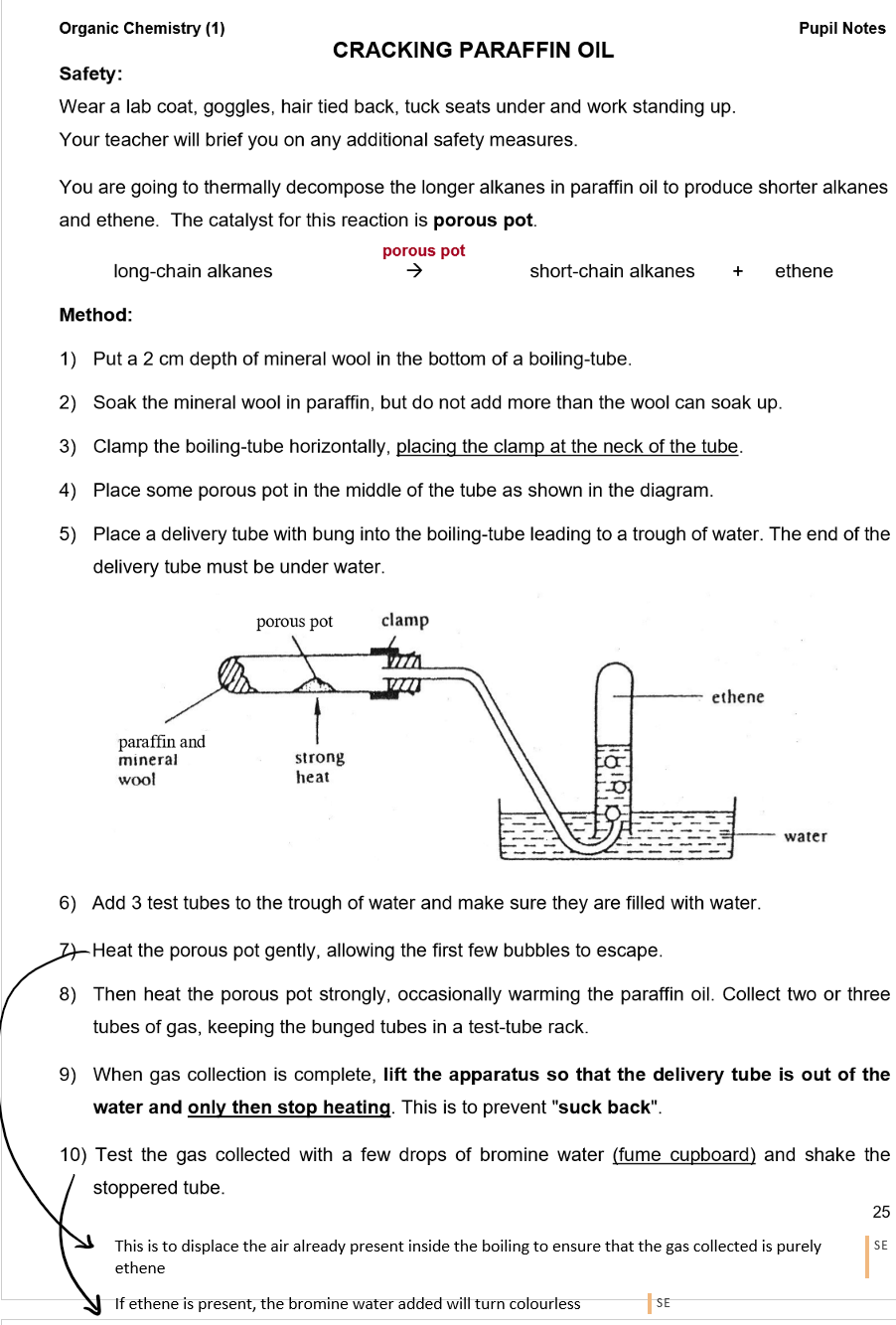
Cracking:
Imbalance in Supply and Demand:
Crude oil contains excess long-chain hydrocarbons (e.g., fuel oil) with low demand.
Short-chain hydrocarbons (e.g., petrol) are in high demand but low supply.
Cracking Process:
Long-chain hydrocarbons are broken into shorter-chain alkanes and alkenes.
Conditions: 600–700°C and a catalyst (Silica or Aluminium oxide).
Cracking is a thermal decomposition reaction.
Uses of Cracking:
Produces short-chain alkanes for petrol.
Produces reactive alkenes for making polymers.
Results in a mixture of products due to random molecule breakage

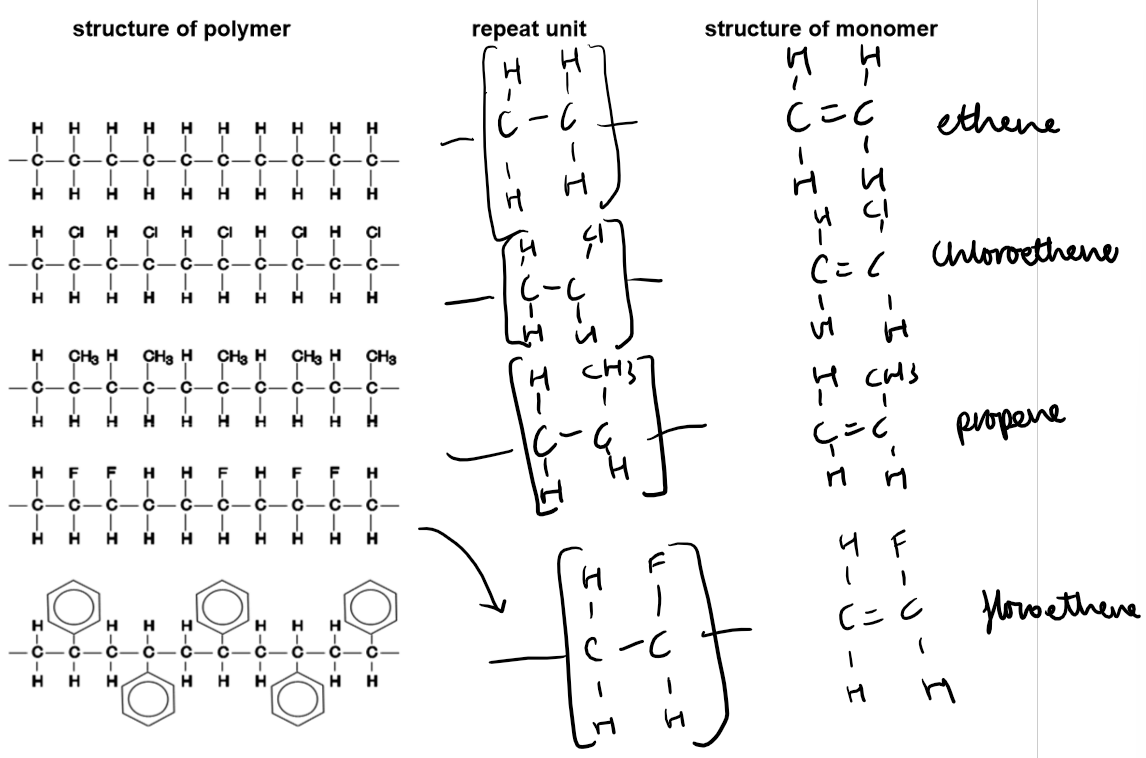
Polymers:
Definition: A polymer is a long-chain molecule formed by joining many small molecules (monomers).
Types of Polymers:
Natural Polymers: Found in nature, e.g., silk, wool, and cotton.
Synthetic Polymers: Made from crude oil, e.g., polyethene, nylon.
Types of Synthetic Polymers:
Addition Polymers:
Formed from unsaturated monomers (alkenes) through addition polymerisation.
Requires heat, pressure, and an initiator (not a catalyst).
Condensation Polymers:
Formed from monomers like dialcohols, diamines, and dicarboxylic acids through condensation polymerisation.
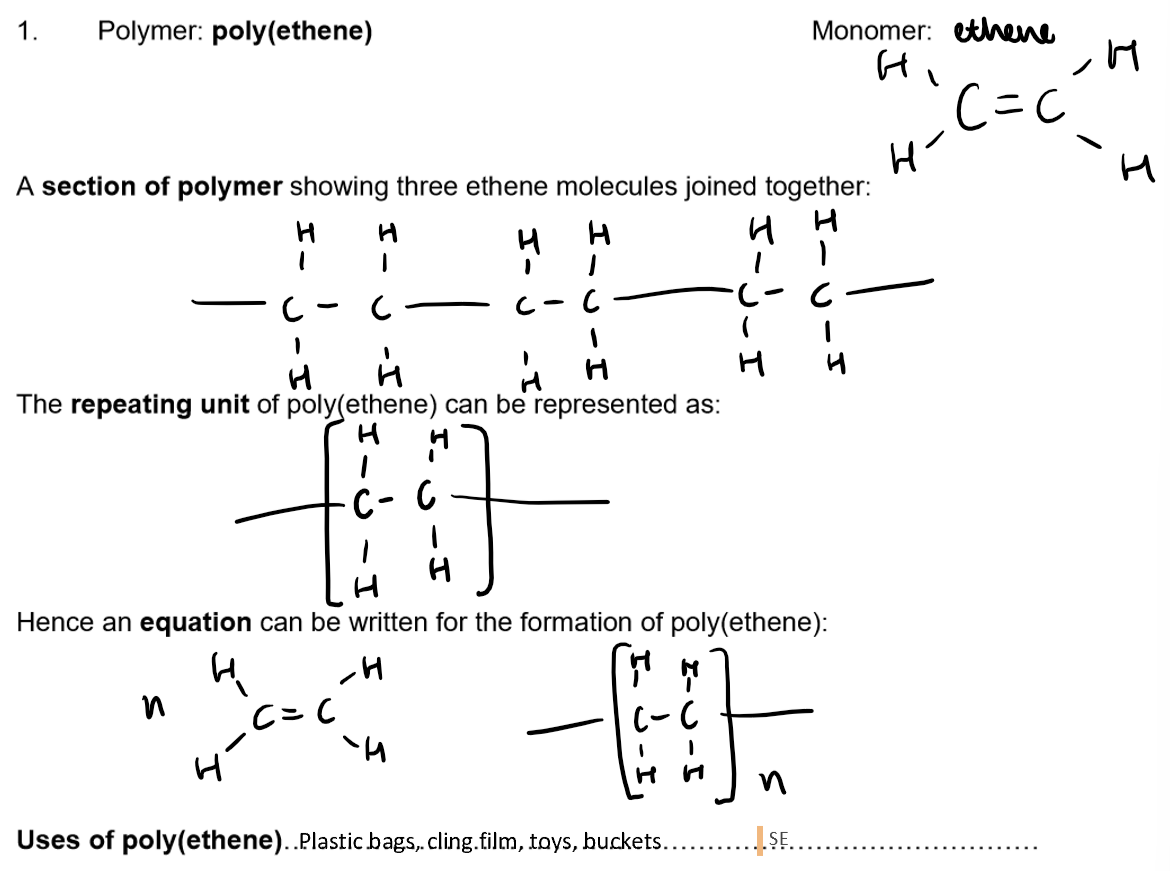
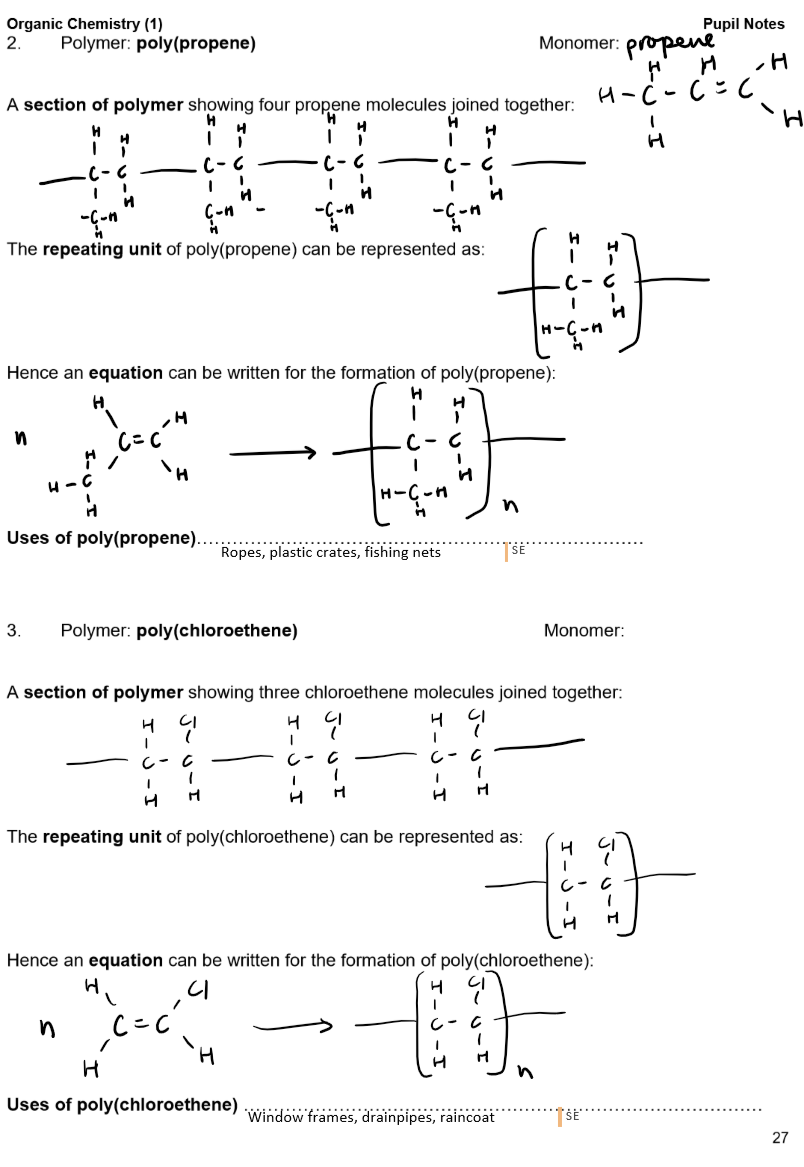
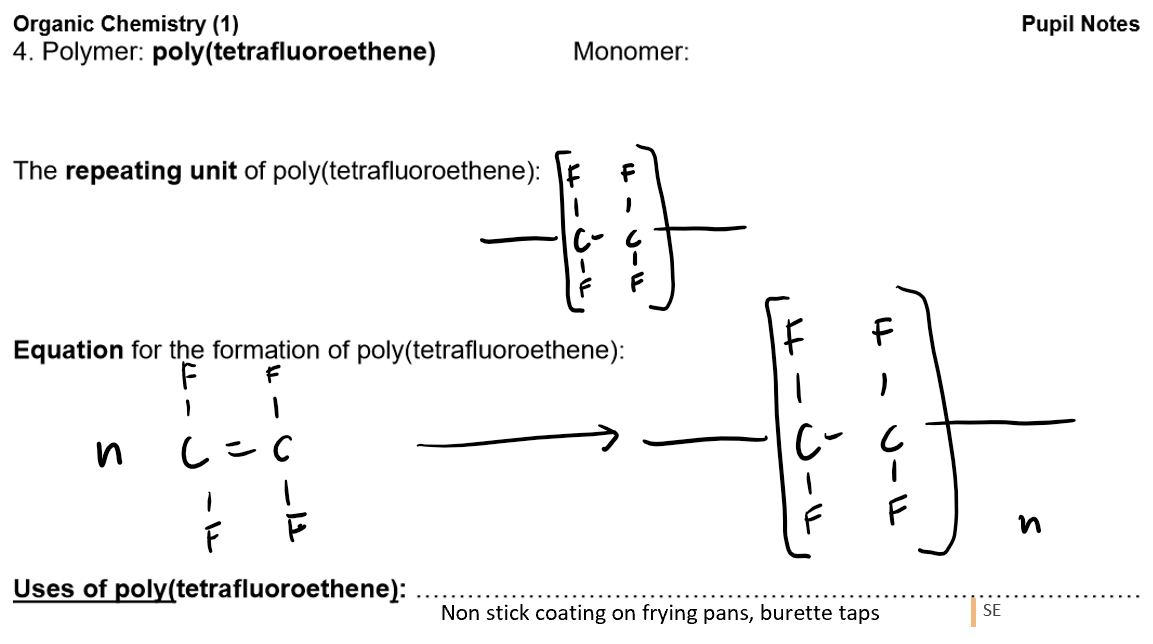
Advantages and disadvantages of plastics (addition polymers)
Advantages:
Corrosion resistant
Good electrical and thermal insulators
Low density and non toxic
Disadvantages:
Inert and non-biodegradable (cannot be broken down naturally or by bacteria)
Some produce toxic gases when burnt (HCN, HCL)
Starting materials come from a finite resource such as crude oil