Slides/AP Videos 3.1-3.7
metabolism - the sum of all chemical reactions occurring in a cell or organism
metabolic pathway - begins with a specific molecule and ends with a product
each step is catalyzed by an enzyme
catabolic pathways release energy by breaking down complex molecules into similar compounds
ex: cellular respiration, the breaking down of glucose in the presence of oxygen,
anabolic pathways consume energy to build complex molecules from simpler ones
the synthesis of a protein from an amino acid is an example of anabolism
enzyme - a catalytic protein - biological catalysts that speed up biochemical reactions
most are proteins
tertiary shape must be maintained for functionality
enzyme names often indicate the substrate or chemical reaction involved
enzyme names often end in -ase
ex) sucrase is an enzymes that digests sucrose
enzymes are reusable
not chemically changed by the reaction
cells typically maintain a specific enzyme concentration
enzymes can facilitate synthesis or digestion reactions
structure is specific resulting in each enzyme only facilitating one type of reaction
catalyst - a chemical agent that speeds up a reaction without being consumed by the reaction
hydrolysis of sucrose by the enzyme sucrase is an example of an enzyme-catalyzed reaction
substrate - the reactant that an enzyme acts on (that enzyme’s substrate)
The enzyme binds to its substrate, forming an enzyme-substrate complex
active site has a unique shape to fit its respective substrate
physical and chemical properties of the substrate must be compatible
small changes can occur to align with substrate
may or may not have chemical charges
active site - the region on the enzyme where the substrate binds
Induced fit of a substrate brings chemical groups of the active site into positions that enhance their ability to catalyze the reaction
*The substrate must have a complementary shape/conformation and charge to its respective enzyme for the reaction to be facilitated
substrate concentration:
initial increases in substrate concentration increases reaction rate
more substrates mean more opportunity to collide with enzyme
substrate saturation will eventually occur
results in no further increase in rate
reaction rate will remain constant if saturation levels are maintained
increased concentration of products decrease opportunity for addition of substrate
matter takes up space
more product in an area means lower chance of enzyme-substrate collisions
slows reaction rate
enzyme concentration:
changes in enzyme concentration can also impact reaction rate
less enzyme = slower reaction rate
less opportunity for substrates to collide with active sites
more enzyme = faster reaction rate
more opportunity for substrates to collide with active sites
activation energy (EA) (free energy of activation) - the initial energy needed to start a chemical reaction
often supplied in the form of thermal energy that the reactant molecules absorb from their surroundings
typically reactions resulting in a net release of energy require less activation energy compared to reactions resulting in net absorption of energy
endergonic - chemical reactions that require a net input of energy
exergonic - chemical reactions that have a net loss of energy
Enzymes catalyze reactions by lowering the EA barrier
enzymes lower the activation energy requirement of all enzyme-mediated reactions, accelerating the rate of reactions
Enzymes are sensitive to local conditions, and under certain circumstances, can lose their shape (denaturation - changes in the conformational shape (of the enzyme))
Denatured proteins are biologically inactive, and for enzymes this means they will not catalyze chemical reactions
Occasionally, denaturation is reversible
Enzyme structure can be affected by general environmental factors, such as temperature and pH
Each enzyme has an optimal temperature in which it can function
range in which enzyme-mediated reactions occur fastest
reaction rates change when optimum temps aren’t maintained
environmental increase in temp - initially increases reaction rate
increased speed of molecular movement
increased frequency of enzyme-substrate collisions
temp increases outside of optimum range result in enzyme denaturation
environmental decrease in temp - generally slows down reaction rate
decreased frequency of enzyme-substrate collisions
does not disrupt enzyme structure, no denaturation
Each enzyme has an optimal pH in which it can function
pH measures the concentration of hydrogen ions in a solution
measured on a logarithmic scale
small changes in pH values equate to large shifts in hydrogen ion concentration
ex) pH 6 has 10x more hydrogen ions in solution compared to pH 7
range in which enzyme-mediated reactions occur the fastest
changing pH outside of this range will slow/stop enzyme activity
enzyme denaturation can occur as a result of increases and decreases outside of optimum
changes in hydrogen ion concentration can disrupt hydrogen bond interactions that help maintain enzyme structure
Optimal conditions favor the most active shape for the enzyme molecule
pH affects enzyme structure due to the increased number of protons in solution
This alters H-bonds in the protein’s structure, causing it to lose its secondary and tertiary structures
Temperature increases the kinetic energy of the enzymes and substrates, increasing collisions and reaction rate (up to a point)
Once the optimum temperature is surpassed, the enzyme will begin denaturing and the reaction rate will decrease
Concentration of substrate and enzymes affect reaction rate as well
Cells produce molecules as needed, and cease producing them when demand is met and homeostasis is restored
Negative feedback allows the cell to avoid wasting energy and resources
Negative feedback is where the product of the pathway inhibits the process responsible for its production
Competitive inhibitors bind to the active site of an enzyme, competing with the substrate
molecules can bind reversibly or irreversibly to the active site of the enzyme
competes with the normal substrate for the enzyme’s active site
if inhibitor concentrations exceed substrate concentrations, reactions are slowed
if inhibitor concentrations are considerably lower than substrate concentrations, reactions can proceed normally
if inhibitor binding is irreversible, enzyme function will be prevented
if an inhibitor binds reversibly, enzyme can regain function once inhibitor detaches
Noncompetitive inhibitors bind to another part of an enzyme, causing the enzyme to change shape and making the active site less effective
do not bind to the active site
bind to the allosteric site
binding causes conformational shape change to the active site
binding prevents enzyme function because the active site is no longer available
reaction rate decreases
increasing substrate cannot prevent effects of noncompetitive inhibitor binding
Allosteric regulation may either inhibit or stimulate an enzyme’s activity
Allosteric regulation occurs when a regulatory molecule binds to a protein at one site and affects the protein’s function at another site
This includes allosteric inhibition or regulation
allosteric site - enzymes can have regions other than the active site to which molecules can bind
According to the first law of thermodynamics, the energy of the universe is constant
Energy can be transferred and transformed, but it cannot be created or destroyed
The first law is also called the principle of conservation of energy
According to the second law of thermodynamics
During every energy transfer or transformation, some energy is unusable, and is often lost as heat
Every energy transfer or transformation increases the entropy (disorder) of the universe
Cells are not in equilibrium; they are open systems experiencing a constant flow of materials
A catabolic pathway in a cell releases free energy in a series of reactions
within a chemical pathway, the product of one reaction can serve as a reactant in a subsequent reaction
the sequential reactions allow for a more controlled and efficient transfer of energy
Metabolic pathways are how cells perform work
Cellular work can be
Transport work
Mechanical work
Chemical work
To do work, cells manage energy resources by energy coupling, the use of an exergonic process to drive an endergonic one
Most energy coupling in cells is mediated by ATP
ATP (adenosine triphosphate) is the cell’s energy currency
ATP is composed of ribose (a sugar), adenine (a nitrogenous base), and three phosphate groups
The bonds between the phosphate groups of ATP’s tail can be broken by hydrolysis
Energy is released from ATP when the terminal phosphate bond is broken
The three types of cellular work are powered by the hydrolysis of ATP
In the cell, the energy from the exergonic reaction of ATP hydrolysis can be used to drive an endergonic reaction
ATP is a renewable resource that is regenerated by addition of a phosphate group to adenosine diphosphate (ADP)
The energy to phosphorylate ADP comes from catabolic reactions in the cell
The ATP cycle is a revolving door through which energy passes during its transfer from catabolic to anabolic pathways
All forms of life must be able to transfer energy from biological macromolecules into usable forms
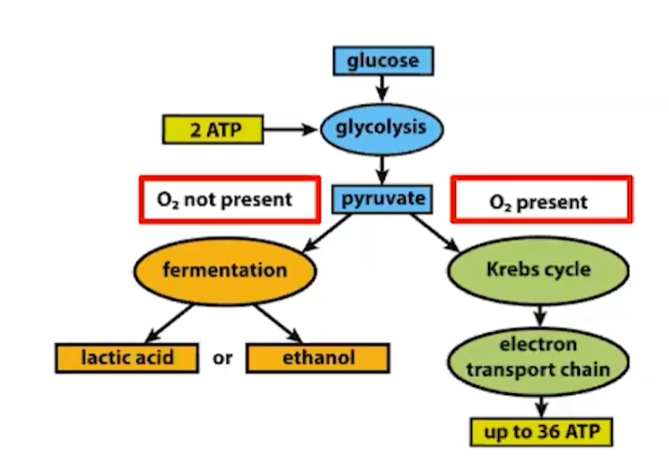
Organisms transfer the energy of organic compounds to ATP through fermentation or cellular respiration
cellular respiration and fermentation are characteristics of all forms of life
Organisms acquire organic compounds for energy in different ways; plants produce their own food while consumers obtain energy by ingesting food
Cells use chemical energy stored in organic molecules to regenerate ATP, which powers cellular work
Catabolic pathways yield energy by oxidizing organic fuels and the breakdown of organic fuels is exergonic
Fermentation is a partial oxidation of sugars that occurs without O2
products are lactic acid or ethanol
Aerobic respiration consumes organic molecules and O2 and yields ATP
Anaerobic respiration is similar to aerobic respiration but consumes compounds other than O2
still have pyruvate (diff from fermentation)
Cellular respiration includes anaerobic and aerobic processes, but is often used to refer to aerobic respiration
Although carbohydrates, fats, and proteins are all oxidized as fuel, it is helpful to trace cellular respiration with the sugar glucose
stages of cell respiration:
Glycolysis (occurs in the cytoplasm)
Pyruvate Oxidation (occurs in mitochondria)
The Citric Acid Cycle (Krebs Cycle) (occurs in mitochondria)
Oxidative Phosphorylation - electron transport chain (occurs in mitochondria)
Oxidative phosphorylation includes an ETC and chemiosmosis
ETC reactions occur in membranes of chloroplasts and mitochondria, and in the cell membranes of prokaryotes
ETC facilitates a series of coupled reactions used during cellular reparation
ETC allow for a more controlled and efficient transfer of energy
ETC use electron energy to establish electrochemical/proton gradient across membranes
Electrons are delivered by electron carriers, aka NADH and FADH2, to the ETC
ATP synthase uses the electrochemical/proton gradient to synthesize ATP
electrochemical gradients are maintained as a result of biological membrane impermeability to charged molecules/ions
oxidative phosphorylation - the process of making ATP using the stored energy of a proton gradient
NADH and FADH2 lose high energy electrons to the ETC = oxidation
ATP synthase adds an inorganic phosphate to ADP resulting in an ATP molecule = phosphorylation
protons moving along the gradient (diffusion), through ATP synthase, powers ATP synthase
energy is stored in proton gradients
decoupling oxidative phosphorylation from electron transport refers to the proton gradient NOT being used by ATP synthase to produce ATP
when decoupling occurs, the energy stored in the gradient is released as heat
the heat from decoupling can be used by endothermic organisms to regulate body temperature
Electrons from organic compounds are transferred to the ETC by an electron carrier, NAD+, in its reduced form, NADH
ATP regeneration can be accomplished in cells via substrate-level phosphorylation or oxidative phosphorylation
Glycolysis and the Citric Acid Cycle make a small amount of ATP using substrate-level phosphorylation
Glycolysis is an ancestral, universal process that occurs in the cytosol
Glycolysis is the splitting of glucose into two pyruvate molecules, producing ATP and NADH
pyruvate is transported from the cytosol to the mitochondrion
pyruvate is actively transported through mitochondrial membranes into the matrix
pyruvate is oxidized and a product of pyruvate oxidation enters the Krebs Cycle
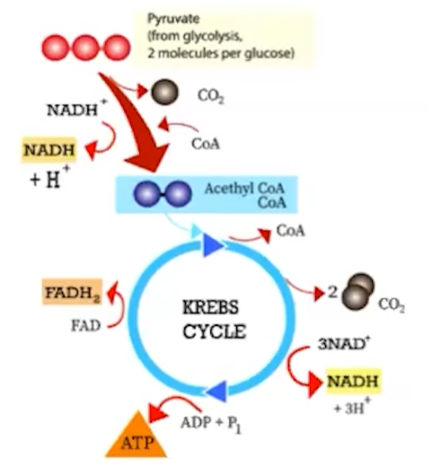
Following the oxidation of glucose, pyruvate is further oxidized into a more reactive compound, Acetyl-CoA
The acetyl-CoA is processed in an enzymatically-mediated cycle called the Citric Acid Cycle or Krebs Cycle
The citric acid cycle has eight steps, each catalyzed by a specific enzyme
in Krebs cycle, carbon dioxide is released from organic intermediates
the Krebs cycle is a pathway involving many key reactions
carbon dioxide is released from intermediate reactions
high energy electrons are transferred to NADH and FADH2
ADP is phosphorylated forming ATP
The NADH and FADH2produced by the cycle relay electrons extracted from food to the electron transport chain
The NADH and FADH2 produced in glycolysis, pyruvate oxidation and the Citric Acid Cycle are oxidized by the proteins of the mitochondrial ETC
NADH created in glycolysis, and NADH and FADH2 created in the Krebs cycle, donate electrons to the ETC
electrons are transferred between membrane proteins of the ETC
ETC establishes an electrochemical gradient of protons (hydrogen ions) across the inner mitochondrial membrane
The mitochondrial ETC is on the highly folded inner membrane
Electrons are passed from one protein complex to the next, until they are accepted by an oxygen atom, the final electron acceptor
As the protein complexes pass electrons, protons are pumped into the intermembrane space
The proton gradient and low pH of the intermembrane space will power chemiosmosis and the production of ATP in large quantities
Fermentation enables cells to produce ATP without the use of oxygen
Without oxygen as the final electron acceptor, the ETC would fail to operate
In the case that oxygen is unavailable, glycolysis couples with fermentation or anaerobic respiration to produce ATP
Anaerobic respiration uses a different final electron acceptor such as sulfate
Anaerobic respiration is a prokaryotic process - ETC protein complexes are embedded in the cell membrane because prokaryotes lack mitochondria
Fermentation includes glycolysis, plus reactions that regenerate NAD+ so that glycolysis can occur again
Two common types of fermentation are alcohol fermentation and lactic acid fermentation
Alcohol fermentation by yeast is used making wine, beer and bread
In lactic acid fermentation, pyruvate is reduced to NADH, forming lactate as an end product without the release of CO2
Human muscle cells use lactic acid fermentation when oxygen is scarce
fermentation allows glycolysis to proceed in the absence of oxygen
ethanol and lactic acid are byproducts of fermentation
the conversion of ATP to ADP releases energy
energy is released when chemical bonds are broken
ATP is converted to ADP when the bond between the 2nd and 3rd phosphate is broken
energy released from ATP hydrolysis can be used to power many metabolic processes
Photosynthesis is the process that converts solar energy into chemical energy
evidence supports the claim that prokaryotic photosynthesis by organisms, such as cyanobacteria, was responsible for the production of oxygen in the atmosphere
photosynthetic pathways are the foundation of eukaryotic photosynthesis
Autotrophs sustain themselves without eating anything derived from other organisms
Autotrophs are the producers of the biosphere, producing organic molecules from CO2 and other inorganic molecules
Photosynthesis occurs in plants, algae, certain other protists, and some prokaryotes
Heterotrophs obtain their organic material from other organisms and are the consumers of the biosphere
Almost all heterotrophs, including humans, depend on photoautotrophs for food and O2
Leaves are the major locations of photosynthesis
Their green color is from chlorophyll, the green pigment within chloroplasts
CO2 enters and O2 exits the leaf through microscopic pores called stomata
The chlorophyll is in the membranes of thylakoids (connected sacs in the chloroplast)
Thylakoids may be stacked in columns called grana
Chloroplasts also contain stroma, a dense interior fluid
Chloroplasts split H2O into hydrogen and oxygen; the electrons from the hydrogen power the production of sugar molecules, and oxygen is released as a by-product
In photosynthesis, electrons flow from water to glucose
Photosynthesis is an endergonic process; the energy boost is provided by light
6 CO2 + 12 H2O + Light energy → C6H12O6 + 6 O2 + 6 H2O
Photosynthesis consists of two stages:
The Light Reactions (the photo- part)
light-dependent reactions capture light energy by using light-absorbing molecules called pigments
The Calvin Cycle (the -synthesis part)
The light reactions, in the thylakoids
Split H2O in a process called photolysis
Release O2
oxygen is produced as a result of water hydrolysis
Reduce NADP+ to NADPH
chemical energy is temporarily stored in the chemical bonds of carrier molecules aka NADPH
Generate ATP from ADP by photophosphorylation
light-dependent reactions help facilitate ATP synthesis
ATP and NADPH transfer stored chemical energy to power the production of organic molecules in another pathway, called the Calvin cycle
The Calvin cycle, in the stroma
Affixes atmospheric CO2 to an organic compound, during Carbon Fixation, using the ATP and NADPH formed during the light reactions
The light reactions convert solar energy to the energy of ATP and NADPH
Light is a type of electromagnetic radiation that occurs in waves
Wavelength determines the type of electromagnetic energy
Pigments are substances that absorb visible light
Different pigments absorb different wavelengths
Wavelengths that are not absorbed are reflected or transmitted
Leaves appear green because chlorophyll reflects and transmits green light
capture energy from sunlight and convert it to high-energy electrons
chlorophyll electrons will be energized
the energy from the electrons will be used to establish a proton gradient and reduce NADP+ to NADPH
An absorption spectrum is a graph plotting a pigment’s light absorption versus wavelength
The absorption spectrum of chlorophyll a suggests that violet-blue and red light work best for photosynthesis
An action spectrum profiles the relative effectiveness of different wavelengths of radiation in driving a process
Chlorophyll a is the main photosynthetic pigment
When a pigment absorbs light energy, its electrons go from ground state to excited state
As electrons return to the ground state, energy is lost in the form of heat and light
A photosystem consists of a reaction-center complex (a type of protein complex) surrounded by light-harvesting complexes
The light-harvesting complexes (pigment molecules bound to proteins) transfer the energy of photons to the reaction center
light-capturing unit in a chloroplast’s thylakoid membrane
referred to as PSII and PSI
the hydrogen molecules from the splitting of water (hydrolysis) are released into the thylakoid space and used to create an electrochemical/proton gradient (which is important for PSII)
electrochemical/proton gradient - a difference in concentration of protons (hydrogen ions) across a membrane
A primary electron acceptor in the reaction center accepts excited electrons and is reduced as a result
Solar-powered transfer of an electron from a chlorophyll a molecule to the primary electron acceptor is the first step of the light reactions
Excited electrons are passed from protein to protein, through the thylakoid membrane, until they reach NADP+, reducing it to NADPH
NADP+ is an electron carrier, a type of molecule responsible for transporting electrons from one cellular chemical reaction to another
1. PSII (P680) is a very strong oxidizing agent, causing photolysis to occur; removing water’s electrons. Oxygen is released and the electrons in PSII are excited by light.
2. Excited electrons are shuttled through an Electron Transport Chain (similar to the ETC in cellular respiration but different location).
PSII and PSI pass high-energy electrons to the ETC (explains how they are functionally related)
3. Electrons reach PSI and are re-excited by light, where they eventually pass to NADP+ and H+, forming NADPH
NADP+ is the final electron acceptor
The NADPH formed will be used to make carbohydrates in the Calvin Cycle
4. While electrons flow through the ETC, protons are pumped from the stroma to the thylakoid space
5. The high proton concentration (and low pH) in the thylakoid space, creates proton motive force, which is required for ATP synthesis
photosynthesis uses a from of passive transport to generate ATP from ADP
6. The enzyme, ATP Synthase, rapidly produces ATP as protons diffuse through it - this process is known as Chemiosmosis
ATP synthase - an enzyme that creates ATP when protons pass through the enzyme
7. The ATP produced will be used in the Calvin cycle
Photophosphorylation is the regeneration of ATP from ADP using the energy of light
The Calvin cycle uses the chemical energy of ATP and NADPH to reduce CO2 to sugar
(Calvin cycle uses ATP, NADPH, and CO2 and produces carbohydrates)
goal - make organic products that plants need using the products from the light reactions of photosynthesis
plants and other organisms mainly get their carbon dioxide from the environment
The cycle regenerates its starting materials after molecules re-enter and leave the cycle
The cycle builds sugar from smaller molecules by using ATP and the reducing power of electrons carried by NADPH
Carbon enters the cycle as CO2 and leaves as a sugar named glyceraldehyde 3-phosphate (G3P)
The Calvin cycle has three phases
Carbon fixation (catalyzed by rubisco)
Reduction
Regeneration of the CO2 acceptor (RuBP)
Organisms have genetic variation allowing them to respond to environmental stimuli
variation can be evident on a cellular and molecular lever
includes differences in…
molecular structure
molecular types, proteins, carbohydrates, lipids, etc.
the number of molecules present
Individuals possessing variations that allow them to survive and reproduce have a higher level of fitness
individual fitness…
refers to an individual organism’s being able to survive and reproduce
contributes to species fitness
not every individual within a species need show fitness for the species to continue generationally
the more variation within individual organisms in a population, the better chance a species can demonstrate fitness generationally under changing environmental conditions
Fitness is a measure of an individual’s reproductive success - organisms that are more fit reproduce more often and pass their genes onto the next generation in greater frequency
A variation that improves reproductive success is also known as an adaptation
Some soil insects can alter the composition of their cell membranes and in cold temperatures to increase the number of phospholipids with unsaturated fatty acids tails
Unsaturated fatty acids enhance membrane fluidity and help prevent the cell from freezing in cold temperatures
Chloroplasts contain multiple types of photosynthetic pigments
This expands the wavelengths of light that the chloroplast can capture and use to produce sugar