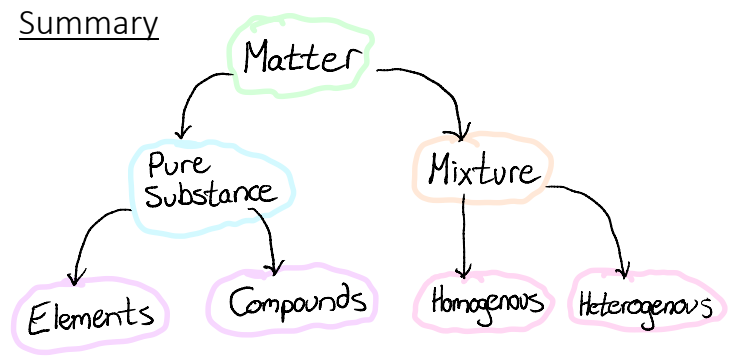
1.1A Elements, Compounds and Mixtures
Elements
A chemical element is a single pure substance, made of only one type of atom.
Atoms of an element are all the same as each other (except for isotopes)
Atoms of an element are different than atoms of another element
Each element is denoted by a chemical symbol

e. g. Gold = Au
An atom is the smallest particle of an element to show the characteristic properties of that element.
Distinct nature of atoms gives each element individual properties
Some elements are found in their native form in nature, uncombined with other elements

Compounds
Other elements are found as compounds, chemical combinations of different elements
Compounds contain fixed proportions/ratios of elements, held together by chemical bonds
Physical and chemical properties of compounds are different than those of the elements they contain (the bonding between atoms changes properties)
e.g. Na Cl2 NaCl
Reactive Toxic Edible
Metal Gas Solid
Mixtures
A mixture is composed of two or more substances in which no chemical combination has occurred
Components have different physical and chemical properties
Composition of mixture is not fixed
Homogenous
Uniform composition and properties
If you take a sample from anywhere in the mixture, you would have the same properties
Heterogenous Mixtures
Non-uniform composition, properties are not the same throughout
It is usually possible to separate components of heterogenous mixtures
Methods of Separating Components
Filtration: process in which solid particles in a liquid are removed through the use of a filter that allows the fluid to pass through but not the solid
Recrystallization: a substance is separated from others by dissolving the mixture in a solvent and then crystalizing the desired substance from the solution
Distillation: components of a liquid mixture are separated through selective boiling and condensation
Paper Chromatography: separates components of a mixture based on adsorption to a solid phase