Biology Chapter 4
Carbon and the Molecular Diversity of Life
Organic Chemistry
Compounds that contain carbon are said to be organic. The study of these compounds is called organic chemistry.
The major elements of life are carbon, hydrogen, oxygen, nitrogen, sulfur, phosphorus. The overall percentages of these elements are equal.
However, carbon is especially important in biology because it can form 4 bonds with one carbon atom. This quality allows carbon atoms to form an inexhaustible variety of organic molecules and compounds.
Carbon Bonds
Carbon has 6 electrons: 2 on the first shell and 4 on the valence shell. In an organic molecule, carbon can form single or double bonds. Each carbon atom acts as an intersection point, from which a molecule can branch off in up to 4 directions. This is what allows carbon to form large and complex molecules.
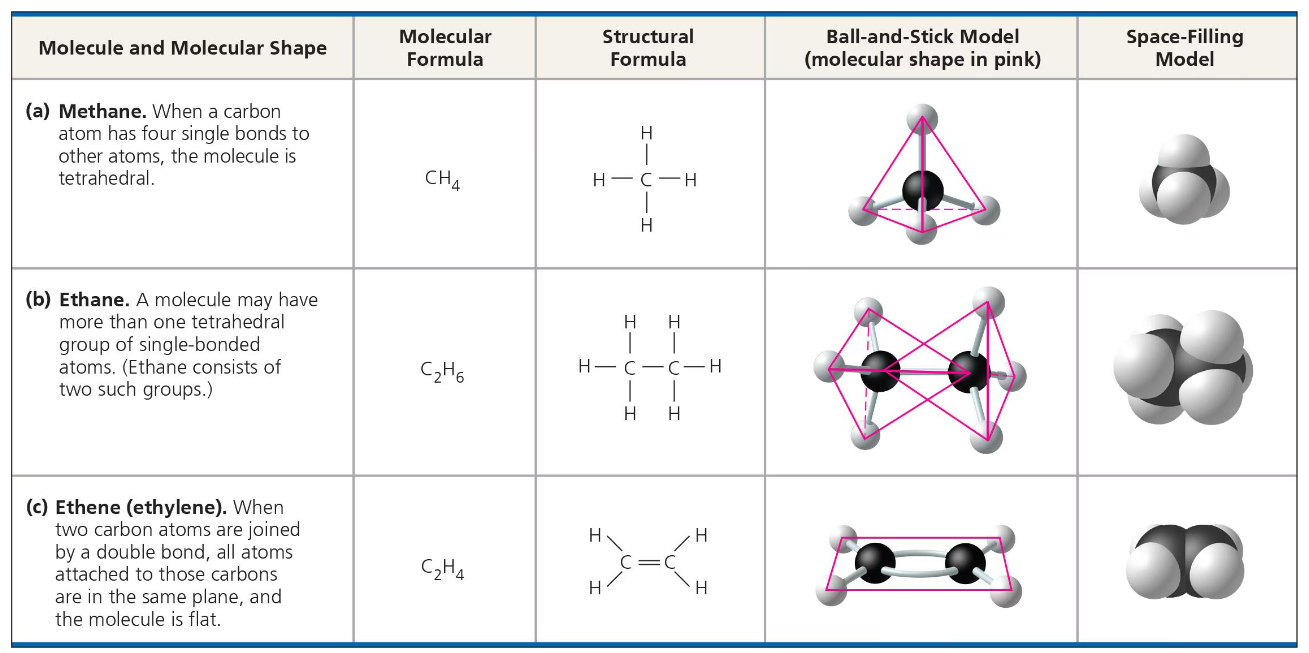
When a carbon atom forms 4 covalent bonds, the arrangement of its four hybrid orbitals will cause the bonds to angle towards the corners of an imaginary tetrahedron.
the bond angles in methane are 109.5 degrees- this is roughly the same for any group of atoms in which carbon has formed 4 bonds.
If there is more than one carbon atom, then there will be that number of tetrahedrons. Ethane, for example, has two carbon atoms so it will have two tetrahedrons
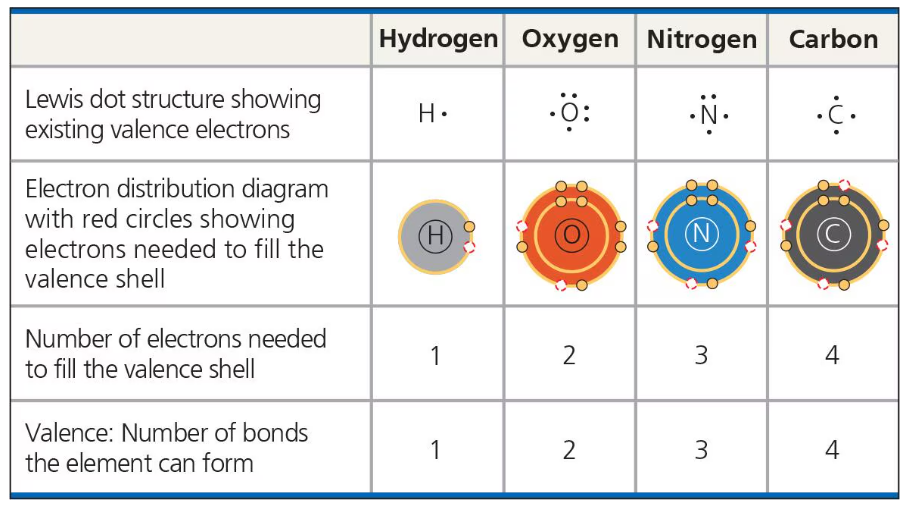
The number of electrons required to fill the valence shell of an atom is equal to the atom’s valence.
The atom’s valence is the number of covalent bonds the atom can form.
These are the valences of the major organic molecules:
The orbital and electron shape (electron configuration) gives it its covalent compatibility with other atoms.
Ex. CO2 (carbon dioxide), carbon is double bonded oxygen on either side, because there are two double bonds, this is the same as four single bonds.
Ex. CO(NH2)2 (urea)
Molecular Diversity in Carbon Skeletons
Carbon chains form the basis of most organic molecules. A carbon skeleton is a carbon chain that helps form a bigger molecule. Carbon skeletons vary in length and may be straight, branched, or arranged in closed rings. Some carbon chains have double bonds which vary in number and location.
Such variation in carbon chains is an important source of molecular diversity and complexity in organisms.
Hydrocarbons
Hydrocarbons are organic molecules consisting of only carbon and hydrogen. In hydrocarbons, hydrogen atoms are attached to the carbon skeleton wherever electrons are available for covalent bonding.
Even though hydrocarbons are not prevalent in living organisms, they do exist in the cells. For example, the molecules known as fats have long hydrocarbon tails attached to a non-hydrocarbon component.
Hydrocarbons usually have nonpolar bonds meaning they do not dissolve in water.
Another characteristic of hydrocarbons is that they can undergo chemical reactions that release relatively large amounts of energy.
In a hydrocarbon, the number of hydrogen atoms is equal to two times the number of carbon atoms plus two.
Hydrocarbons are called reduced molecules. A reduced molecule is a molecule that contains high amounts of potential energy. As we will later learn, fats contain lots of hydrocarbons and this potential energy is what allows them to provide us energy.
Isomers
Isomers are molecules with the same molecular equation (the same number of atoms of the same elements) but are different molecules with different properties because they have different structures. There are three types of isomers:
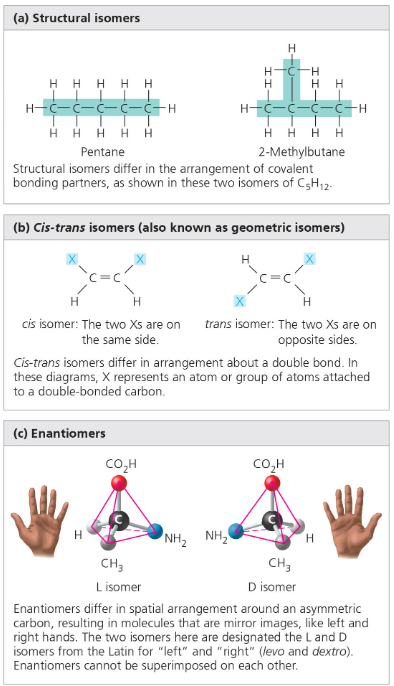
Structural isomers: These isomers differ in the covalent arrangements of their atoms. The number of possible isomers increases as the size of the carbon skeleton increases. For example, C5H12 only has three possible isomers, while C8H18 has 18 isomers.
ex. Two structural isomers of C5H12 are pentane and 2-Methylbutane. Pentane has a straight carbon skeleton while 2-Methylbutane has a branched carbon skeleton.
Glucose and fructose are structural isomers. Both have the same molecular formula of C6H12O6, but glucose is an aldose sugar while fructose is a ketose sugar.
Cis-trans isomers: These isomers have the same covalent arrangements, but the order of these arrangements may be different. Cis-trans isomers must have a double bond between the two carbon atoms. A cis isomer has the same atom bonded to each carbon on each side, while in trans isomers, two of the atoms are switched. While these differences may seem small, they make a huge difference in the properties of the molecules. For example, an example of a trans isomer is a trans-fat.
Enantiomers (stereoisomer): Enantiomers are isomers that are basically a mirror image of each other. This is due to the presence of an asymmetric carbon. An asymmetric carbon is a carbon that is attached to four different atoms or atom groups. These four groups can be arranged around a carbon in two different ways. The concept of enantiomers is especially important in the pharmaceutical industry because 2 enantiomers of a drug will have different effects.
Ex. Methamphetamine is a drug that has two enantiomers. One enantiomer is a highly addictive stimulant that is sold illegally through drug dealers. The other enantiomer has a much weaker effect and is an active ingredient in over-the-counter vapor inhalers.
Any molecule that has an asymmetric carbon will have a stereoisomeric form. An asymmetric carbon is a carbon that has four different groups bonded to each side.
The only amino acid that does NOT have a stereoisomeric form is Glycine. This is because its R group is a hydrogen molecule. All 19 other amino acids have an isometric form.
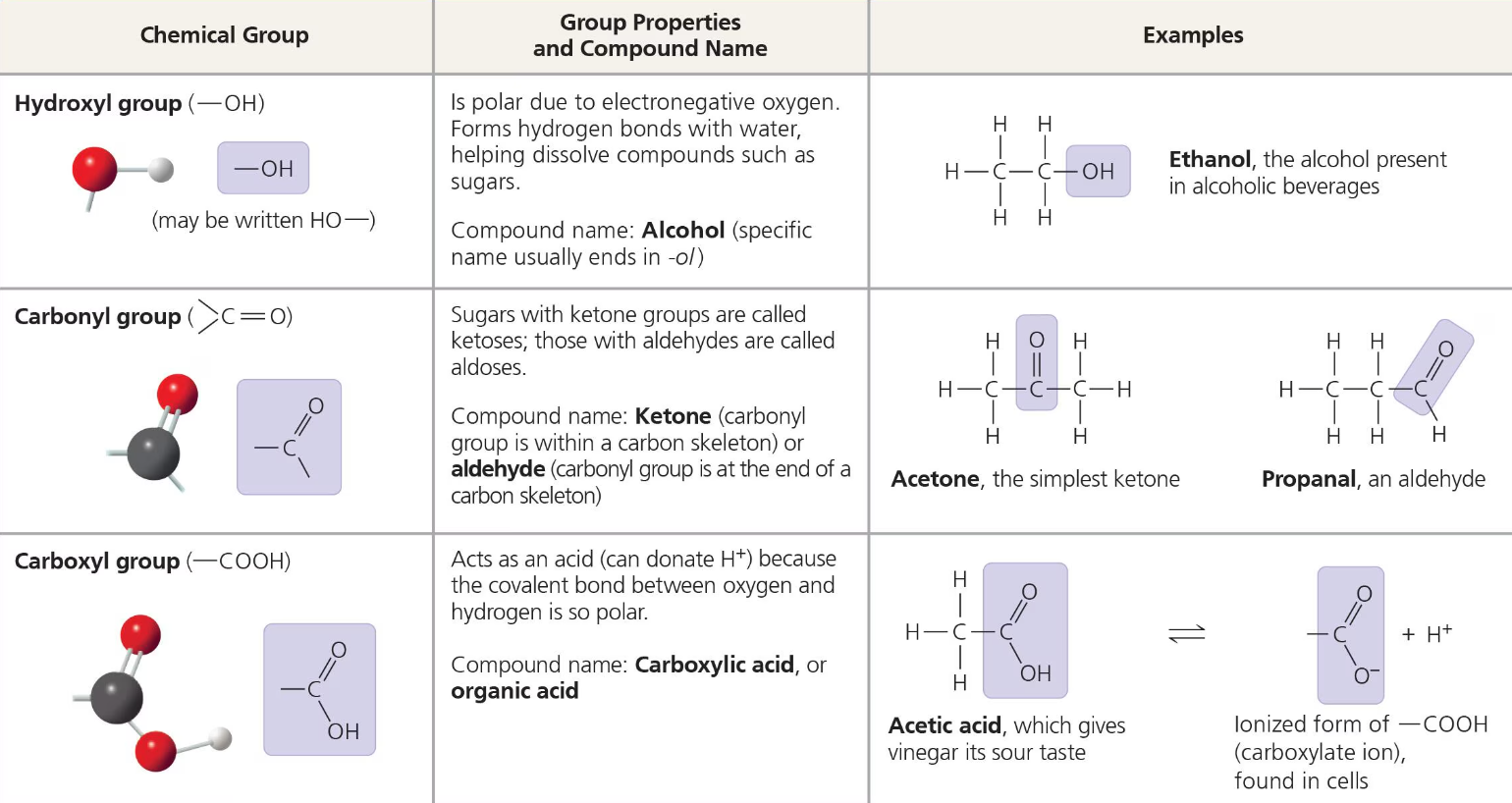
Most Important Chemical Groups of Life
Chemical groups are extremely important because they can make a big difference in the properties of two otherwise similar molecules.
Ex. Estradiol (a type of estrogen) and testosterone are both steroids with a common carbon skeleton (four fused rings). The only difference are the chemical groups attached to the rings; estradiol has a HO at the end, while testosterone has an O and an additional CH3. These seemingly small differences completely change the functions of these two molecules. One is the female sex hormone while the other is the male sex hormone.
In this case, chemical groups are important because they change the structure of a molecule therefore changing its function.
In other cases, however, chemical groups are directly involved in the chemical reactions of molecules. These groups are called functional groups. Each functional group has different properties such as shape and charge which can help it participate in a chemical reaction.
Functional Groups
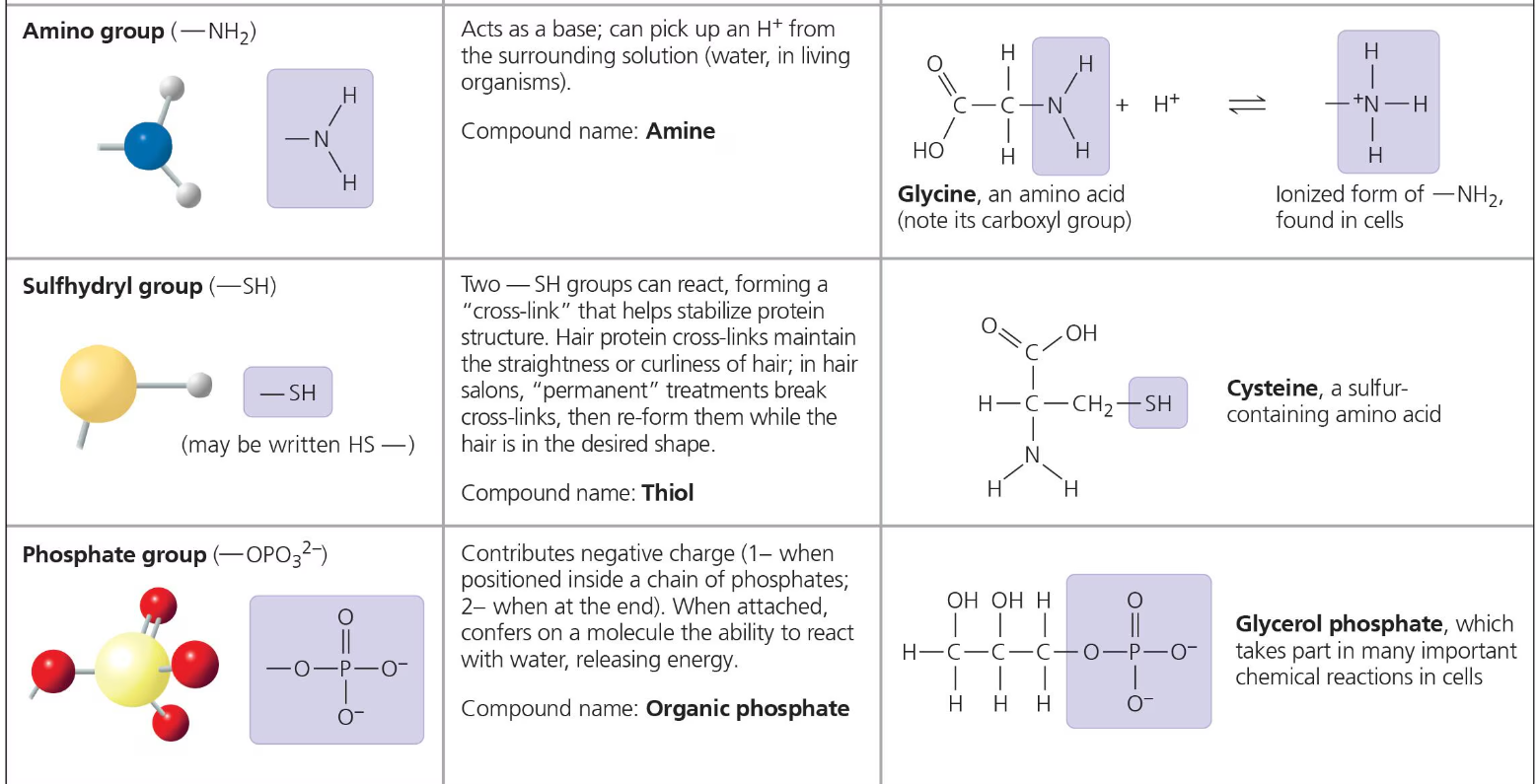
The seven functional groups that are most important in biological processes are: the hydroxyl group, carbonyl group, carboxyl group, amino group, sulfhydryl group, phosphate group, and methyl group.
The first six of these groups are chemically active and, excluding sulfhydryl, they are hydrophilic and thus increase the solubility of their molecules as well.

ATP
ATP or adenosine triphosphate is an example of a complex molecule with a phosphate group. Like the name suggests, it consists of a molecule called adenosine bonded to three phosphate groups. When ATP is added to water, one of the phosphates may split off, making the molecule adenosine diphosphate. This allows ATP to release energy.