( Chapter 6 - Metabolism 09.25.25)
¬ ˚ This topic spans over two days and covers the key developments that occurred during this period.
day 1 - metabolism, energy (kinetic, potential)
day 2 - ender/exergonic, ATP cycling, redox reactions, the role of enzymes,
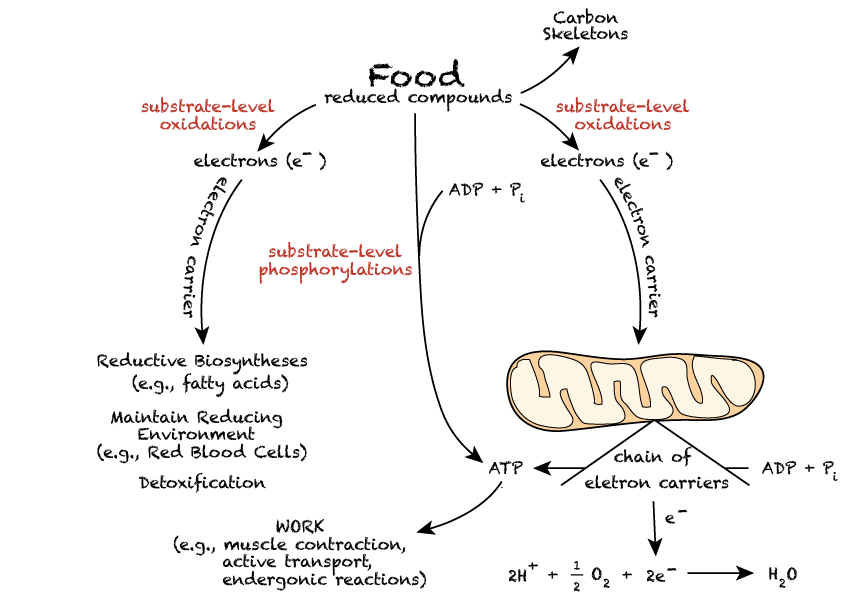
✦ Metabolism - the sum of all chemical reactions within a cell or organism
Sometimes common to discuss metabolism with energy
metabolism requires energy, which is the capacity to perform work or cause a change
:: kinetic energy - light, radiant heat, and motion; all of which play a role in driving metabolic processes.
ex: roller coaster. The ride continues to move fast on its own after it’s given motion by the machine that helps it. The massive loops and dramatic turns add to this factor.
bat, gas stove (on), bike, roller coaster, dolphin, power lines, sun, power plant, playing instruments, volcano erupting, atoms fusing, sun rays
:: potential high or low and chemical. The stored energy in an object due to its position, state, or arrangement
ex: a fire hydrant or a car. The hydrant is immobile, meaning that at some point it will be able to move due to all of the water being stored. When it’s acted on, it becomes kinetic because it moves on its own.
soup, coal, tree, car, iron, rainbow, batteries, fire hydrant, bell, electrons, gasoline, bowling balls & pins, geiger counter
law of thermodynamics - entropy
chemical reactions either store or release
∆G = ∆G Final state (products) - G initial state (reactants)
day 2
✦ ATP shuttles chemical energy within the cell. It can be used as a readily available unit of energy for the cell to do work. For that to happen, it needs to undergo a process called
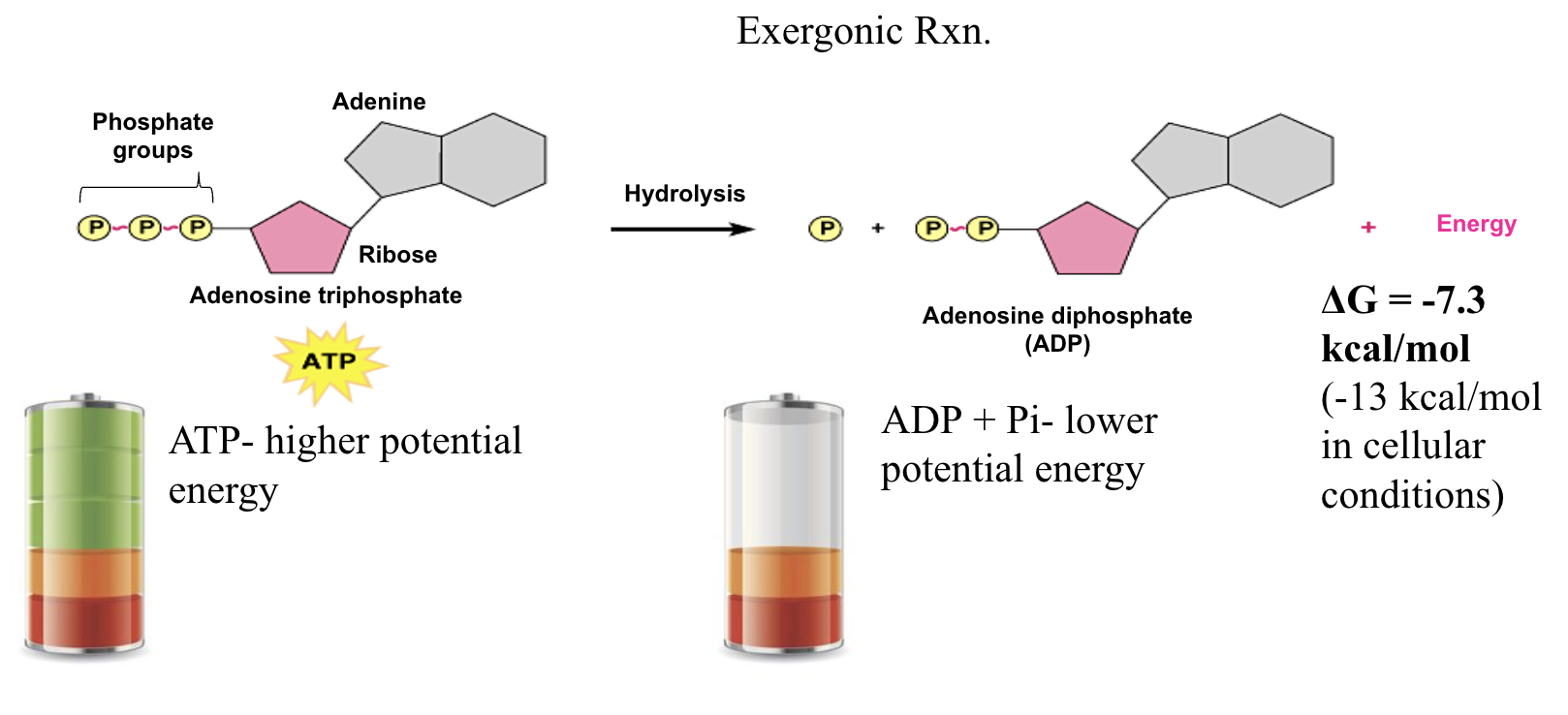
The structure of ATP (adenosine triphosphate) and its hydrolysis into ADP (adenosine diphosphate) and an inorganic phosphate group (PiPi), which is the process by which ATP releases chemical energy for cellular work.
When ATP is converted to ADP, there is a loss of energy.
+ The ∆G is negative.
The amount of energy released is 7.3 kcal/mol available to do work (THE PHOTO IS MISTYPED)
Below is an example of CYCLING through ATP and ADP, illustrating how the energy currency of the cell is regenerated and utilized:

Energy input would be an endergonic reaction. In the photo above, that would be where energy from food is.
Energy output would be the exergonic reaction. In the photo above, that would be where energy for cells is released.
:: Coupling - exergonic reactions can provide energy for simultaneous endergonic reactions. This process is essential for sustaining cellular functions, allowing organisms to perform vital activities such as muscle contraction and biosynthesis.
An exergonic reaction could fuel an endergonic reaction and vice versa.
PRACTICE
Given the ΔG for ATP hydrolysis is -7.3 Kcal/mol, which of the following endergonic reactions would ATP be able to fuel? Why?
A + B → C (3.2 Kcal/mol)
G + E → F (8.0 Kcal/mol)
X + Y → Z (6.5 Kcal/mol)
Given that the for ATP hydrolysis is -7.3 Kcal/mol, ATP would be able to fuel endergonic reactions 1 and 3.
When an exergonic reaction (like ATP hydrolysis, which releases 7.3 Kcal/mol) is coupled with an endergonic reaction (which requires energy), the overall change in free energy (\Delta G) must be negative for the coupled reaction to proceed spontaneously.
A + B → C (3.2 Kcal/mol)
Coupled \Delta G = +3.2\text{ Kcal/mol} + (-7.3\text{ Kcal/mol}) = -4.1\text{ Kcal/mol}
Since the net \Delta G is negative, ATP can fuel this reaction.
G + E → F (8.0 Kcal/mol)
Coupled \Delta G = +8.0\text{ Kcal/mol} + (-7.3\text{ Kcal/mol}) = +0.7\text{ Kcal/mol}
Since the net \Delta G is positive, ATP cannot effectively fuel this reaction.
X + Y → Z (6.5 Kcal/mol)
Coupled \Delta G = +6.5\text{ Kcal/mol} + (-7.3\text{ Kcal/mol}) = -0.8\text{ Kcal/mol}
Since the net \Delta G is negative, ATP can fuel this reaction.
Therefore, ATP hydrolysis provides enough energy to make reactions 1 and 3 energetically favorable when coupled, but not reaction 2.
✦ REDOX REACTION - A redox reaction involves the transfer of electrons between two species, resulting in:
oxidation (loss of electrons)
reduction (gain of electrons)
This process is also coupled ~ Xe- + Y → X + Ye-
:: NADH is an electron carrier. Cycles between an oxidized and reduced form. This allows NADH to shuttle electrons during cellular respiration, facilitating ATP production in the mitochondria (cellular respiration).
NAD+ is the oxidized form. In order to reduce this, two electrons and a H+ are required.
:: NADPH is the reduced form of NADP+, which plays a crucial role in anabolic reactions, particularly in the Calvin cycle of photosynthesis.
✦ Reactions in a cell are primarily catalyzed by enzymes and can be part of a metabolic pathway, which is a series of interconnected biochemical reactions that convert substrates into products, helping to regulate energy flow and metabolic processes within the cell. Below is a simplified example of a metabolic reaction.
A →Rxn1 B → Rxn2 → C Rxn3 → D
Each enzyme in those reactions is different. In this case, A would be the reactant, B and C would be the intermediates, and D would be the final product
✦ Enzymes - biological catalysts that increase the rate of chemical reactions without being consumed in the process, enabling the efficient transformation of substrates into various metabolites.
Lower activation energy, speeding up reactions. Once a reaction is started, it’ll continue until all reactants are used up. However, enzyme activity can be influenced by factors such as temperature, pH, and substrate concentration, which can either enhance or inhibit their effectiveness.
PRACTICE
Identify the optimum temperature on this graph
In terms of enzyme activity, what is going on at the optimum
Explain low activity below the optimum temperature
Explain low activity above the optimum temperature
✦ Enzyme Inhibition - The process by which the activity of an enzyme is reduced or prevented, often by substances called inhibitors that interfere with the enzyme's ability to bind to its substrate or catalyze the reaction. It can be competitive or noncompetitive.
competitive inhibition: a molecule other than the substrate competing with the substrate for the active site
✦ Noncompetitive Inhibition - A type of inhibition where the inhibitor binds to an allosteric site, altering the enzyme's shape and thus reducing its effectiveness, even when the substrate is bound.
Metabolic pathways can be regulated by feedback inhibition.
Too much of a product can inhibit an earlier step in the pathway, preventing the overproduction of that substance and maintaining homeostasis.
