1.1B Kinetic Theory
All matter can exist in different forms called states of matter depending on the temperature and pressure
States of matter are characterized by the energy of the particles; the average kinetic energy is directly related to the temperature of the system
Temperature: a measure of the average kinetic energy (Ek)of the particles of a substance
State of matter at a given temperature and pressure is determined by the strength of the inter-particle forces (forces between the particles)
Solid | Liquid | Gas | |
Diagram | |||
Packing | Particles close packed | Particles more spaced | Particles spread out |
Inter-Particle Forces | Strong (more attraction) | Weaker | Negligible |
Particle Motion | Particles vibrate in position | Particles slide over each other, vibrate, rotate, translate | Particles move freely, vibrate, rotate, translate (faster than liquid) |
Shape | Fixed shape | No fixed shape (takes shape of container) | No fixed shape (expands and occupies available space) |
Volume | Fixed volume | Fixed volume (don't compress easily) | No fixed volume |
Liquids and gases are called fluids, which refers to their ability to flow. (Reason why liquids take the shape of their container)
Diffusion: process by which particles of a substance become evenly distributed, as a result of random movement
Kinetic energy (Ek): the energy associated with movement or motion
Ek=1/2 mv2
Kinetic energy of particles at same temperature is equal
This gives inverse relationship between mass and velocity
m = mass in grams
v = velocity in m/s
higher mass -> lower velocity
Changes in State
Kinetic energy increases with temperature
As particles move faster, they overcome inter-particle forces and change state
State changes occur at a fixed temperature and pressure for each substance
Sublimation: direct inter-conversion solid to gas without going through the liquid phase. Characteristic at atmospheric pressure of some substances like dry ice.
Deposition: reverse of sublimation, changes from gas to solid
(e. g. Frosting)
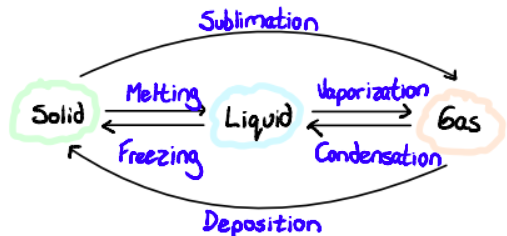
Vaporization
Vaporization is the change in state of matter from liquid to gas. It can be accomplished through evaporation or boiling
Evaporation
Evaporation: change in state that occurs only at the surface of a liquid and at a temperature below the boiling point.
When heat is absorbed from the surroundings, particles at the surface acquire enough kinetic energy to vaporize and escape
Boiling
Boiling: volume phenomenon, happens throughout a liquid, characterized by particles leaving throughout the body of the liquid, forming bubbles
Boiling point: when all molecules in a liquid have enough kinetic energy to change into a gas.
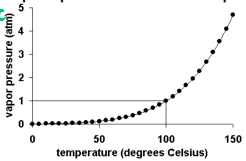
Vapor Pressure
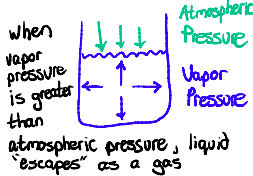
Vapor Pressure: the pressure exerted by a liquid on the walls of a container
During evaporation, vaporization is slow because the vapor pressure is less than atmospheric pressure
Boiling occurs when vapor pressure = atmospheric pressure