AP Chem Unit 1
The Mole and Molar Masses
The mole
The mol is a counting unit in chemistry
6.022×10²³ units
also called alvagaldos number
defined as the number equal to the number of carbon atoms in exactly 12 grams of pure carbon -12
meaning that the whole period table is based on carbon -12
Using the mole In calculations
atomic value (amu) found on the table tells us how many grams are in one mole of that element
Ex:
how many moles of copper are. in a penny with a mass of 3.14g
start with 3.14 g- turn into moles with the molar mass
to turn into atoms → turn moles into the counting unit
Molar Mass
mass in grams of one mol of the compound (g/mol)
how to find
1. account for every mole of each compound found in the element
turn the moles into grams using the average atomic masses given on the table
Mass Spectroscopy and Average Atomic Mass
atomic masses are based on carbon-12 as the standard
c-12 will have exactly 12 amu
Mass Specroscopy
an instrumental method that identifies the chemical constitution of a substance by separating it into gasious ions and based on mass and chchrge
used to find the relative abundance and the atomic/molar mass of an unknown sample
y-axis is the relative abundance of particles
x-axis is the m/z or mass over charge
usually one electron is taken away so the x axis just represents the mass
Steps:
vaporization - substances must be gasses
ionization - knocking off electrons (usually just one)
Accelleration - to have equal KE
Deflection - a magnetic field will deflect them, the lighter they are, the more they are deflected, leading them to a sharper turn
Detection - detector plate detects ions
Average Atomic Mass - the weighted average of the masses of the naturally occurring isotopes for that element
are located on the periodic table
how to calculate average atomic mass when given percents and mass
multiply the percents in decimals times the atomic mass
How to use spectrometer graph
it will provide relative abundances and mass number, calculate based on that
Percent Composition of Compounds and Determining the Formula of a Compound
there are 2 ways to describe the composition of a compounds
Percent Composition by mass
can be determined by comparing the mass of each element present in 1 mole of the compound to the total mass of 1 mole of the compound
mass percent of the element = (mass of element in 1 mole of compound)/ (mass of 1 mole of compound) times 100%
example:
Find the percent composition from formula of N2O5
we know that one mole of dinitrogen pentoxide has 2 moles of nitrogen and 5 moles of oxygen
we find the mass of each element using stoichiometry to turn moles of the element to grams
then we find the total mass of the compound
then we use the formula for the mass percent of the element found above
NO SIG FIGS because moles are exact.
Example: what if we were given a substance in grams that yielded elements in grams
just divide the elements in grams over the substance before in grams
Always make sure everything is in grams to find percent composition in grams
Another Situation:
Formula of a Compound
Molecular Formula: is the exact formula that gives the type of atoms and the number, this is only for nonmetals
Formula unit: for ionic compounds, is always empirical, so it. is the lowest ratio
Emperical formula: simplest ratio of a formula and can be calculated with the percent composition’s
how to get empirical formula from percent composition
ex: Ba: 69.58%, C: 6.090%, O: 24.32%
Turn the percents into grams but pretend it was from 100 grams
then with the grams of each element, turn into moles through stociometry
then divide by the smallest moles
multiply all until you get 0.9 or 0.1 from a full mol
that is how many moles of each element are in the empirical formula
what if there. is a missing percent? then you just subtract. the ones given from 100%too find the missing one
what if I was given grams from a sample rather than percents? then turn grams into moles. and continue on with the steps
Always turn to moles
How to turn empirical into the molecular formula
find the molar mass of the empirical formula
divide the molar mass (given) over the empirical formula mass
the value given will be the number to multiply by all the empirical formula to get the correct molecular formula
Emperical Formula from Combustion
combustion is the burning of a hydrocarbon in presence of oxygen and → CO2 and H2O
ex: given grams of original sample, given yielded CO2 and H2O in g
turn grams of CO2 and H2O into moles of C and H
g → moles CO2 → 1molC/1mol CO2 → moles C
Then find grams of C and H and subtract that from the sample to find mass of O
mass of O → moles
Dicide by smallest mole, then multiply to get full mole number
if given the actual molar mass → Molar mass/emperical mass → coefficient
Polyelectronic Atoms, The Aufbau Principle, and the Periodic Table
Polyelectronic Atoms - is an atom having more than one electron
Aufbau Principles - an electron occupies the lowest energy orbital that can receive it
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s
use the table
Perioid Table Blocks
Tells us what the last electron filled is
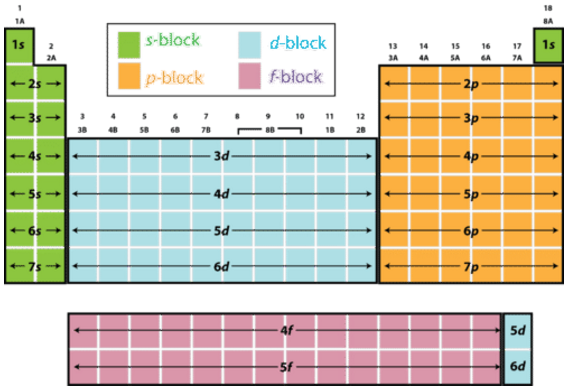
Electron Confifuration - is how the elcetrons are distributed among the various atomic orbitals in an atom
1s² (the 1 is the pinricpal quantum number), S is the type of subshell? and the exponent is the number of e-
Shorthand Notation - find the closest noble gas that has less electrons
mark the noble gas with []
resume the electron config
Orbital Diagram
Hunds Rule: when filling orbitals with electrons, each orbital in a subshell should be singly occupied before any orbital is doubly occupied
Pauli's Exclusion Principle states that no two electrons in the same atom can have identical values for all four of their quantum numbers.
meaning that no more than two electrons can occupy the same orbital and (2) two electrons in the same orbital must have opposite spins
Exceptions to Audbau Principle:
d and f orbitals require a lot fo energy
d^4 and d^9 exceptions
they are one electron short of being held full
to become stable (less energy), they take an e- from the closest s orbital
Ions in Electron Config
Electrons come out from the highest energy level not the orbital
ex:
Atom: [Kr] 5s2 4d10 5p2
Sn+2 ion: [Kr] 5s2 4d10
Sn+4 ion: [Kr] 4d10
Photoelectron Spectroscopy
used to understand atomic structure, electron config, ionization energy, and periodic trends
What is PES?
the photoelectron spectrophometers use high energy radiation photon to eject (ionize?) electrons from an atom
bc electrons are at different energy levels, they require different amounts of energy
valence easy, inner core are harder
What is measured?
the photon energy removes the electron, the left over KE energy determines how fast the electron is moving
ifthe electron is slow, it means it required a lot of ionization energy to remove the electron
if. theelctron is fast, a lot of KE, it means little energy was required to remove the electron
Reading the Graphs
Y axis - signal intensity, number of electrons or relative number of electrons
X- axis: represents the ionization energy or the binding energy
it is. backward x axis
to the left, means lots of energy to remove e-, probably inner elections
to the right, it is little energy to remove the valence electrons
Periodic Trends
Periodic Table
was originally constructed to represent the patterns observed in the properties of elements
the first chemist was Dobereiner with a triad model
John Newlands Octave model
Meyer and Mendeleev - atomic mass
Mendeleev was given the most credit because he was able. to predict the existence and properties of unknown elements like atomic masses
current periodic table is made by Henry mosley, based on the atomic number
Valence Electrons - are the outermost energy. level electrons
elements with similar valence configuration show similar chemical behavior
in the main group representative elements, the groups have the same electron config
predicting the valence electron config of transition metals
Core Electrons are the inner electrons
Columbic Attraction: the positive and negative attraction
Effective Nuclear Charge is the pull that an electron “feels” from the nuclear
(Zeff) = # protons - # core electrons
the closer an electron is to the nuclear, the more pull it feels
as the effective nuclear charge increases, the electron cloud is tighter
Ionization Energy - the energy required to remove an electron from a gasous atom or ion
X (g) + energy → X+(g) +e-
the highest energy electron (the one bound least tight) is removed first
when removing electrons from ions, it becomes harder because there is a larger proton to electron ratio
when removing from a full shell, the energy is greater by a lot
left to right first ionization increases because more protons in the nuclear (higher effective nuclear charge) because more protonts and smaller radius
first ionization energy decreases in going down a group because the electrons removed are farther because in a different energy level from the nucleus, lower columbic attraction “feel lesss of nuclear force”
Atomic Radius
the radius is defined as hald the distance between the nucli in a molecule consisting of identical atoms
decrease when going left to right: because the increasing effective nuclear charge and the valance electrons are drawn closer
increases down a group: because of the increases in the obrital size bc more level
Size of Ions
Negative ions are always larger than the atoms of what they are formed
this is because of electron repulsions between them increase and electrons push apart and occupy more volume
and lower protein pull compared
Positive Ions are always smaller than the atoms from they are formed
because the electrons are removed from valence electron and electron repulsions decrease and the elcetrons pulled together close
also because the electrons pull them closer
Electronegativity - is a measure of the ability of an atom in a chemical compound to attract electrons
valence electrons hold atoms together in chemical compounds
some compounds have valance electrons concentrated closer to one atom than another and this uneen concentration affects the properties of a compound
increases across each period
decreases or stays the same down the period
most electronegativ is flourine
Electron Affinity - the amount of energy involved when an electron is accepted by a gasous atom. tofodm a negative ion
“neutral atoms likelihood of gaining an electron”
Metals don’t want to gain electron to be stable so it might be endothermic or very low
Nonmetals want an electron
Halogens require energy to receive
increases left. toright
decreases as you go down