IB Chemistry SL Organic Lesson 1-5 Review
Lesson 1: Homologous Series
A homologous series is a family of compounds that differs only by the length of its hydrocarbon chain. (Ex: Alkanes - CH4, C2H6, C3H8)
Members share:
General formula
Chemical properties
As you go down a homologous series, chain gets longer, the boiling point increases: Gas → Liquid → Solid.
Types of Formulas
Empirical formula - smallest whole number ratio.
Molecular formula - actual number.
Full structural formula - displayed formula. Drawn structure and arrangement of atoms.
Condensed Structural Formula - Similar to molecular formula but in order of bonds.
Lesson 2: Isomers (and naming)
Compounds with the same molecular formula but different structural formulas. (Same number of each atom, but bonded in a different order).
Naming
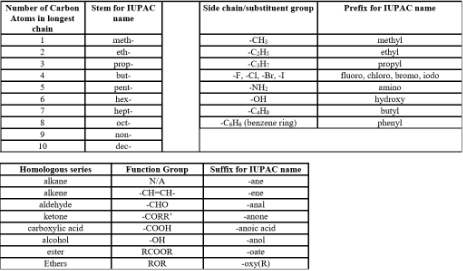
Lesson 3: Meet the Families
Alkanes
Alkanes really don’t do much.
Combustion is one of two notable reactions, hence, we use them for fuels.
Complete combustion:
Alkane + O2 → CO2 + H2O
Incomplete combustion:
Alkane + O2 → C + CO + CO2 + H2O
Alkanes are stable because they have full valence shells and high bond enthalpies.
Halogenation
Alkanes will undergo halogenation if reacted with a halide in the presence of U.V. light.
C2H6 (g) + Cl2 (g) → CH3CH2Cl (g) + HCl (g)
Radicals
Radicals are species with unpaired electrons.
Halogens form radicals when hit by U.V. light of the right frequency.
Cl2 → 2Cl*
The asterisk represents the unpaired electron.
This process is called homolytic fission - the bond breaks equally with one electron going to each chlorine.
Free Radical Substitution
Cl2 + uv → 2Cl*
Initiation - radicals formed by homolytic fission.
Cl* + C2H6 → C2H5 + HCl
C2H5* + Cl2 → C2H5Cl + Cl*
Propagation - these steps feed each other the radicals needed to continue.
Cl* + Cl* → Cl2
Cl* + C2H5* → C2H5Cl
C2H5* + C2H5* → C4H10 Termination - any two radicals can combine to terminate the reaction.
Crude Oil
We get residual fuel oil, lubricating oils, diesel, kerosene, naphtha, gasoline blending components, and refinery gases from crude oil.
Lesson 4: Molecular Modelling
Lewis Structure
Count valence electrons.
Determine central atom.
Most unpaired electrons.
Least electronegative.
Add surrounding with bonding pair - subtract electrons from total.
Subtract octets from total.
Make multiple bonds to complete octets.
VSEPR
Group of negative charge will repel one another - negatively charged centers / electron domains.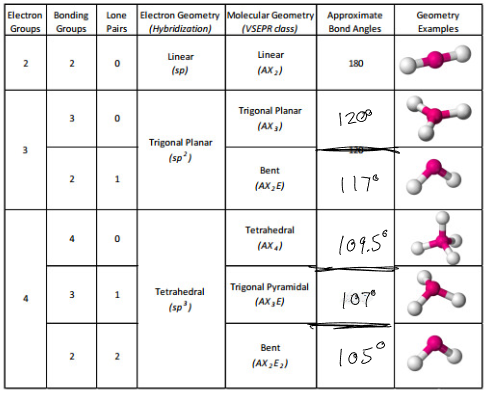
Lesson 5: Alkenes
Alkenes are considerably more reactive that alkanes and are a major industrial feedstock.
The reactivity is due to the double bond:
The double bond contains 4 electrons.
This is a significant amount of charge which:
Makes it attractive to electrophiles.
Enables it to polarise approaching molecules.
Most reactions of alkenes are addition reactions. All reactions remove the double bond.
Alkenes and hydrogen
Alkene + hydrogen (H2) → alkane
Reaction conditions:
Hot >120°
Ni catalyst
Alkenes and hydrogen halides
Alkene + hydrogen halide (H-Br) → halogenoalkane
Reaction conditions:
This reaction occurs very readily and needs no special conditions.
Alkenes and halogens
Alkene + halogen (Cl-Cl) → dihalogenoalkane
Reaction conditions:
This reaction occurs very readily and needs no special conditions.
Standard test for alkenes:
Halogen used in aqueous solution of bromine becomes decolourised.
Polymerisation
Alkene + alkene → Polymer
Reaction conditions:
Vary from alkene to alkene but often include high pressure, temperature, and a catalyst.
Alkenes and water
Alkene + water (H2O) → alcohol
Reaction conditions:
Water must be steam.
Phosphoric or sulphuric acid catalyst.