Periodic Trends
Atomic Radiuse
decreases across a row
REASON: nuclear charge is increasing pulling those electrons in closer to the nucleus
the distance of electrons from the nucleus gets smaller because it has more electrons
increases down a column
the shell and the principle quantum number of the shell is getting larger
the electrons are farther from the nucleus
Measure of radius
Diffraction technique or from bnod length of a diotomic molecule
half of the bond length is the atomic radius
Importance of atomic radius: selective ion channels in any cells where a very rapid signaling is important
MORE CHARGE=MORE FORCE
Ionization Energy
To form a positive ion, an electron must be removed from a neutral atom. This requires energy. The energy is needed to overcome the attraction between the positive charge of the nucleus and the negative charge of the electron.
*usually refers to the first ionization energy
Ionization Energy = final energy of the products - the energy of the reactants; energy required to remove an electron from a gaseous atom
first ionization energy = energy to remove the electron from the highest occupied atomic orbitation
second ionization energy= energy to remove the 2s electron for example in boron plus
third ionization energy= remove the next strongly bound electron
Left to right = ionization energy increases
the nuclear charge is increasing
when the nuclear charge increases the Coulomb interaction is greater bringing electrons closer to the nucleus (more strongly bound)
putting electrons into the same shell
on the average, the distance of those electrons from the nuclelus is about the same
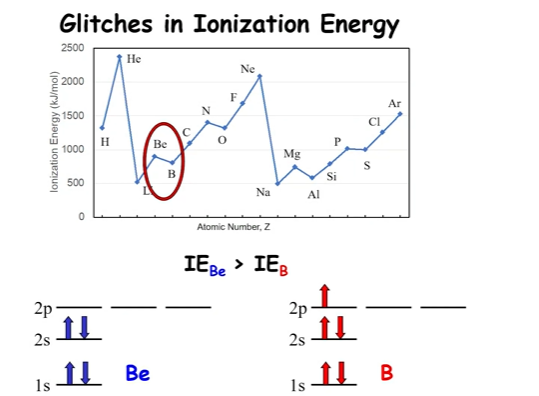
*2p is higher than 2s in a multi electron atom
the nuclear charge isn’t high enough to overcome the extra energy to access the 2p state
As you go down
the ionization energy decreases
the principal quantum number is increasing because the shell is larger
the distance from the electron from the nucleus is larger
Electronegativity
essentially the average of the ionization energy and the electron affinity; indicates the relative ability of its atoms to attract electrons in a chemical bond
high electronegativity= good electron acceptor
high electron affinity
the energy released in adding an electron is large
located in upper right hand corner on the right hand side of the periodic table
increase as you go from left to right
Low electronegativity=electron donor
electron affinity is low value
located in the lower left hand corner of the periodic table
decreases as you move down
high x low electronegativity = ionic bonds
want to attract the electron from the lower corner
Ionic Radius
isoelectronic= having the same electron configuration
Relative size of the radii of ions vs their neutral partners
- 40% decrease in the radius
if you add an electron to iodine to form iodine radius
increase in about 55% in the atomic radius