Titration Refresher!
Titration
A titration is a laboratory procedure used to determine the concentration of a solution using a standardized solution (a solution with a known concentration.
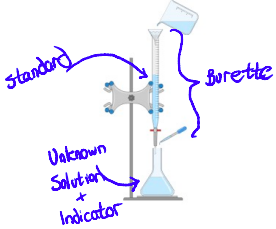
Main materials
Burette
Unknown solution
Standard solution
Indicator
Indicators
An indicator is a chemical that has different colours in its dissociated and undissociated state. An indicator’s color depends on the pH of a solution. The point at which an indicator changes colour is called the endpoint.