Chapter 1: DNA
DNA structure
DNA is composed of two polynucleotide chains
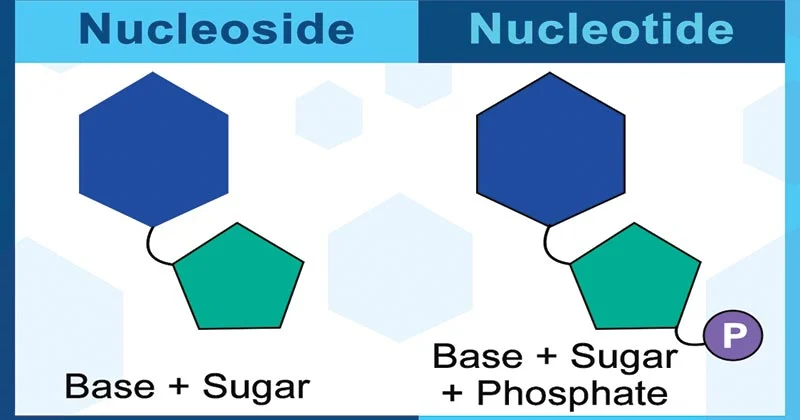
nucleoside:: 2’-deoxyribose + base
nucleotide:: phosphate + nucleoside
phosphodiester linkage:: phosphoryl group between 2 nucleotides has one sugar esterified through 3’-OH and a second sugar esterified through 5’-OH
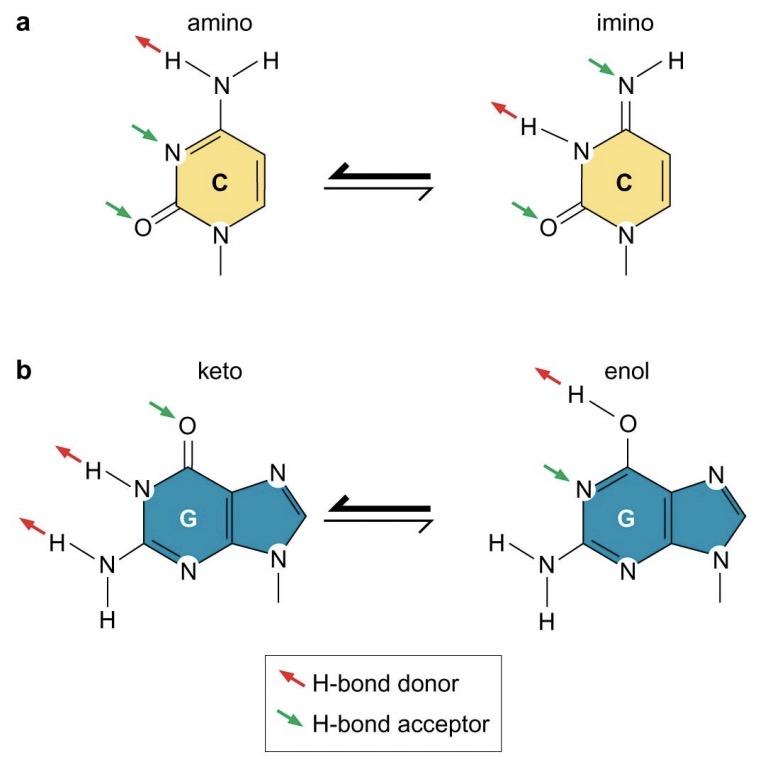
Each base has its preferred tautomeric form
DNA bases:
purines ~ adenine, guanine (double ring)
pyrimidine ~ cytosine, thymine (single ring)
Base tautomers:
N attached to purine and pyrimidine rings normally have the amino form and rarely take on the imino configuration
O attached to G and T normally have the keto form and rarely take on the enol configuration
The two chains of the double helix have complementary sequences
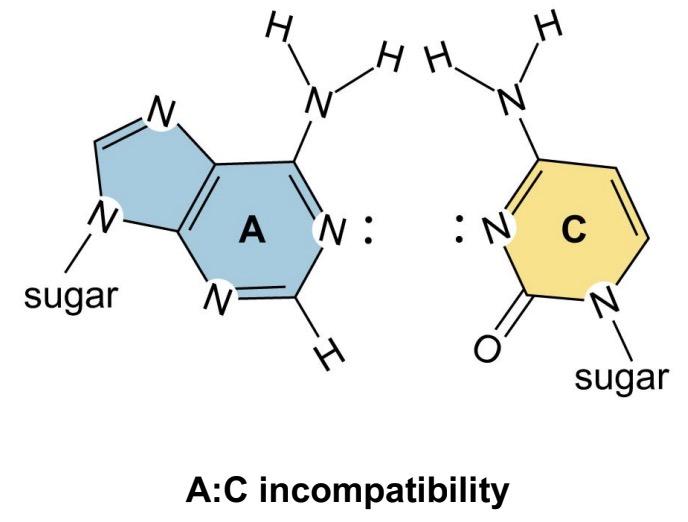
“Watson-Crick” pairing results in a complementary relationship between sequence of bases (A-T, G-C) and gives DNA its self-encoding character
All base pairs accommodate within the same arrangement with no distortion of the overall structure of DNA
Non-Watson-Crick base pairs
G:U, G:A are most common
allows enhanced capacity for self-complementarity
Stability of the double helix
hydrogen bonding (correct match is meaningful)
H bonding is important for the specificity of base pairing
hydrophobic effect
Interacting with water
increase in entropy
hydrophobic interactions between bases provide driving force for DNA to form double helix
base stacking
base pairs are stacked and within van der Waals contact distance
large number of van der Waals interactions significantly increase stability of double helical structure
stacking energy is more stabilizing for complementary dinucleotides containing at least one G-C base pair (due to more favorable interactions)
DNA is usually a right-handed double helix
10.5 bp / turn
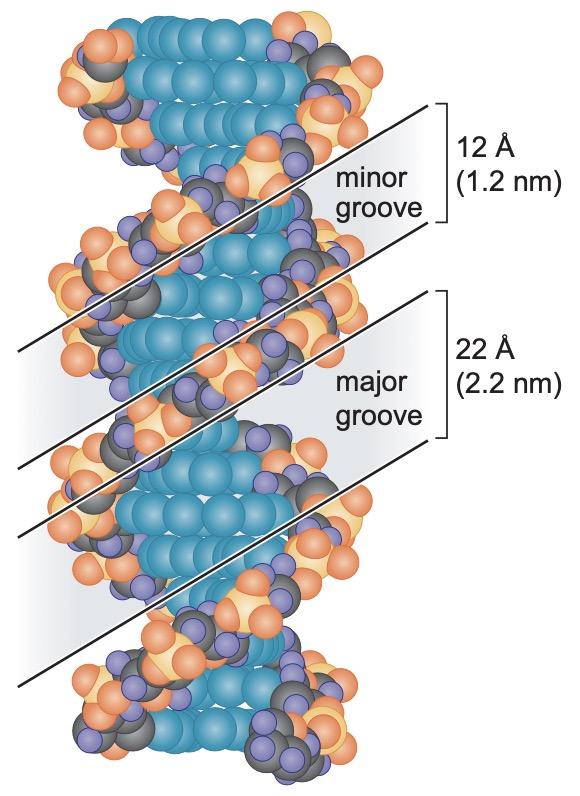
the backbone strands are closer in the minor groove and further in the major grooves
Major groove specifies the identity of the base pair -> allows proteins to recognize DNA sequences without disrupting the double helix
AADH = G:C base pair (major)
HDAA = C:G base pair (major)
ADAM = A:T base pair (major)
MADA = T:A base pair (major)
A…A (minor) is used to recognize correct base-pairing
the minor groove is not rich in useful chemical information
The more G-C, the harder it is to unwind because G-C has the highest stacking Energy and higher melting temperature.
Proteins that recognize DNA sequence
Protein structure can adjust slightly to create complementarity with a slightly altered base sequence
GCN4
binds to DNA only when the sequence at the contact site is correct
side chains are complementary in shapes, polarity, and H-bonding properties to the DNA surface
bacteriophage λ repressor
symmetric dimer with N-terminal DNA-binding domain and C-terminal dimerization domain
a helix-turn-helix motif fits against DNA so that the ‘recognition helix’ fits into the major groove of DNA
zinc-finger proteins
Determine the sequence of the DNA
most abundant DNA-recognition domain
becomes ordered when it binds to DNA
a hydrophobic core, and zinc ion in the center holds the folded domain together
lymphocyte enhancer factor-1 (LEF-1)
regulates T-cell gene expression
Fits into widened minor groove
influence DNA to bend or twist
Regulation of DNA-binding proteins
Lac repressor causes the two domains in the body of the repressor to change orientation with respect to each other
The double helix exists in multiple conformations
B DNA (majority)
real DNA is never perfectly regular: a ‘propellar twist’ -> width of major and minor groove varies locally
A DNA (without water)
more compact
large tilt of base pairs with respect to the helix axis
has central hole
similar to the structures of RNA-DNA, RNA-RNA helices
Z DNA
alternating purine and pyrimidine residues assume left-handed conformation
A DNA | B DNA | Z DNA | |
|---|---|---|---|
Overall proportions | Short, broad | Long, thin | Elongate, wider |
Helix rotation | Right-handed | Right-handed | Left-handed |
Bp / turn | 11 bp / turn | 10.5 bp / turn | 12 bp / turn |
Helix axis location | Major groove | Through bp | Minor groove |
Major groove | Narrow, deep | Wide | Flattened out on helix surface |
Minor groove | Broad, shallow | Narrow | Very narrow, deep |
Glycosyl-bond conformation | Anti | Anti | Anti at C, syn at G |
Observed at | low humidity conditions in X-ray | high humidity conditions in X-ray |
Syn and anti conformations:
always anti in right-handed DNA
repeating purine-pyrimidine residues in left-handed DNA have glycosidic bonds in alternating anti (:pyrimidine) – syn (: purine) conformations
G tetraplex is the exception of the antiparallel concept of DNA.
DNA strands can separate and reassociate
by heat or high pH
Hybridization - forming hybrids between complementary strands of DNA and RNA
DNA maximally absorbs UV light at ~260 nm primarily due to its bases
base stacking in duplex DNA diminishes capacity to absorb UV
temperature of DNA solution near to the boiling point of water increases capacity to absorb UV (hyperchromicity)
melting temperature of DNA is largely determined by the G:C content and the ionic strength of the solution
sequences rich in G-C base pairs are more stable due to more favorable base stacking interactions
at high ionic strength, negative charges of the backbone phosphoryl groups prevent the repel between the two DNA strands -> stabilize helix
DNA topology
Linking number is an invariant topological property of cccDNA
linking number (Lk)
number of times one strand would have to be passed through the other strand in order for the two strands to be entirely separated from each other
can only be changed by breaking one or both strands of the DNA, winding them tighter or looser, and rejoining the ends
Supercoiling
relaxed DNA (free)
Lk0: linking number in relaxed DNA = (number of bps) / (average bp/turn)
Supercoiling
DNA structure is strained in a manner that is regulated by the cell to induce supercoiling
linking difference (∆Lk= Lk - Lk0)
∆Lk < 0 (underwinding) -> negative supercoiling (right-handed)
aids strand separation
can be converted into untwisting of double helix
∆Lk > 0 (overwinding) -> positive supercoiling (left-handed)
prevent DNA denaturation
Cells in extremely hot water tend to have a positive writhe
superhelical density (σ = ∆Lk / Lk0)
normalized measure of supercoiling
σ of circular DNA purified from cells is ~ (- 0.06)
Overtwisting is more common because translation and transcription go constantly
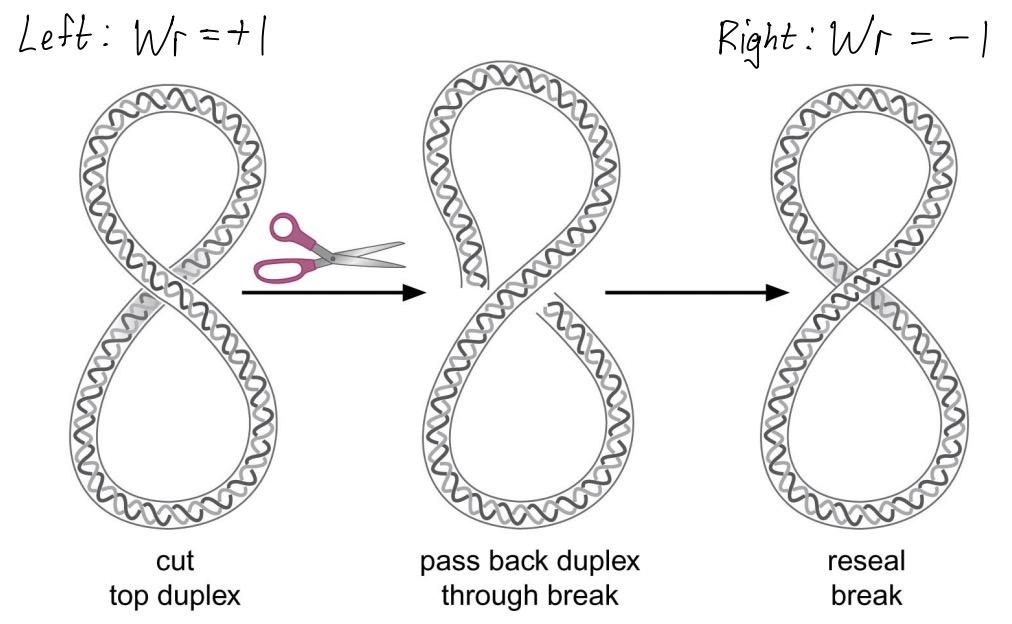
Twist and writhe
Lk = Tw + Wr
twist (Tw) - number of turns
writhe (Wr) - supercoiling
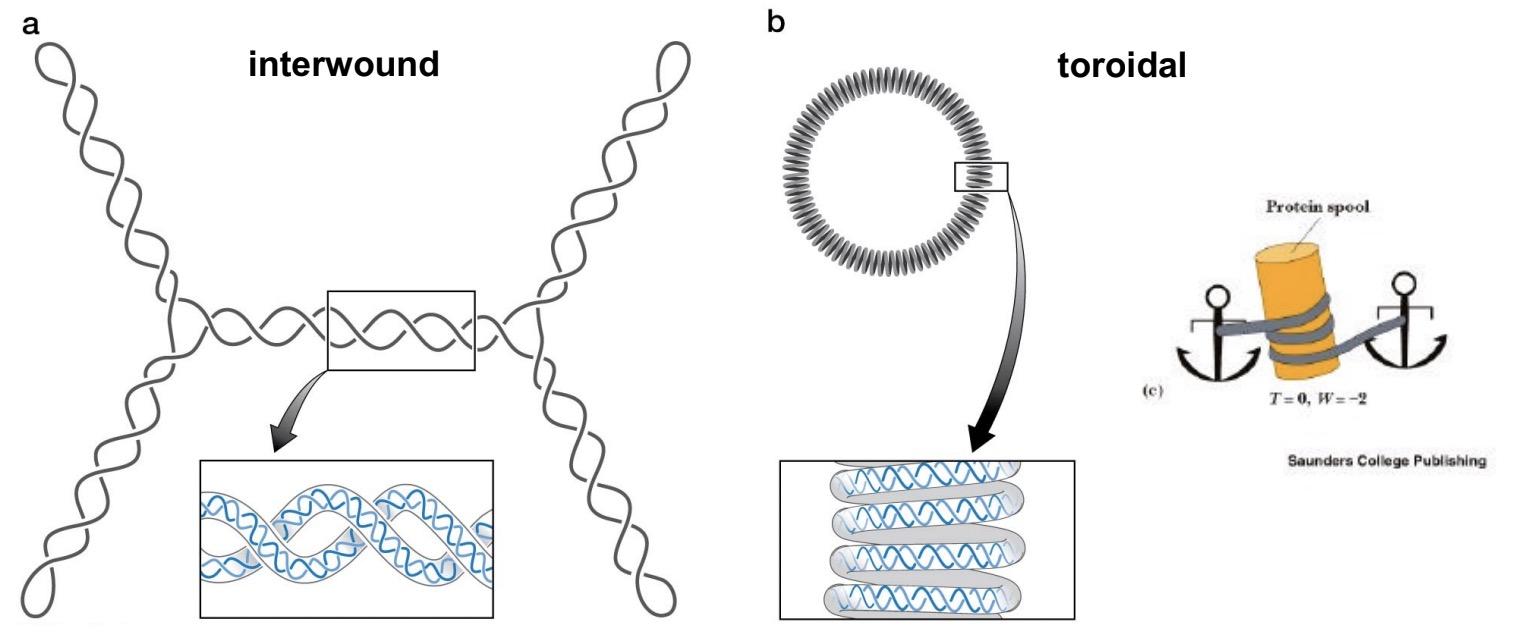
interwound (plectomic) writhe
twisted around itself (supercoiling)
~ 40% of the length of the DNA
toroid (spiral) writhe
a cylindrical manner
occurs when DNA wraps around protein
Both forms are interconvertible
in nucleosomes, DNA is wrapped around a protein core in a left-handed manner -> equivalent to negative supercoiling (negative σ )
Topoisomerases
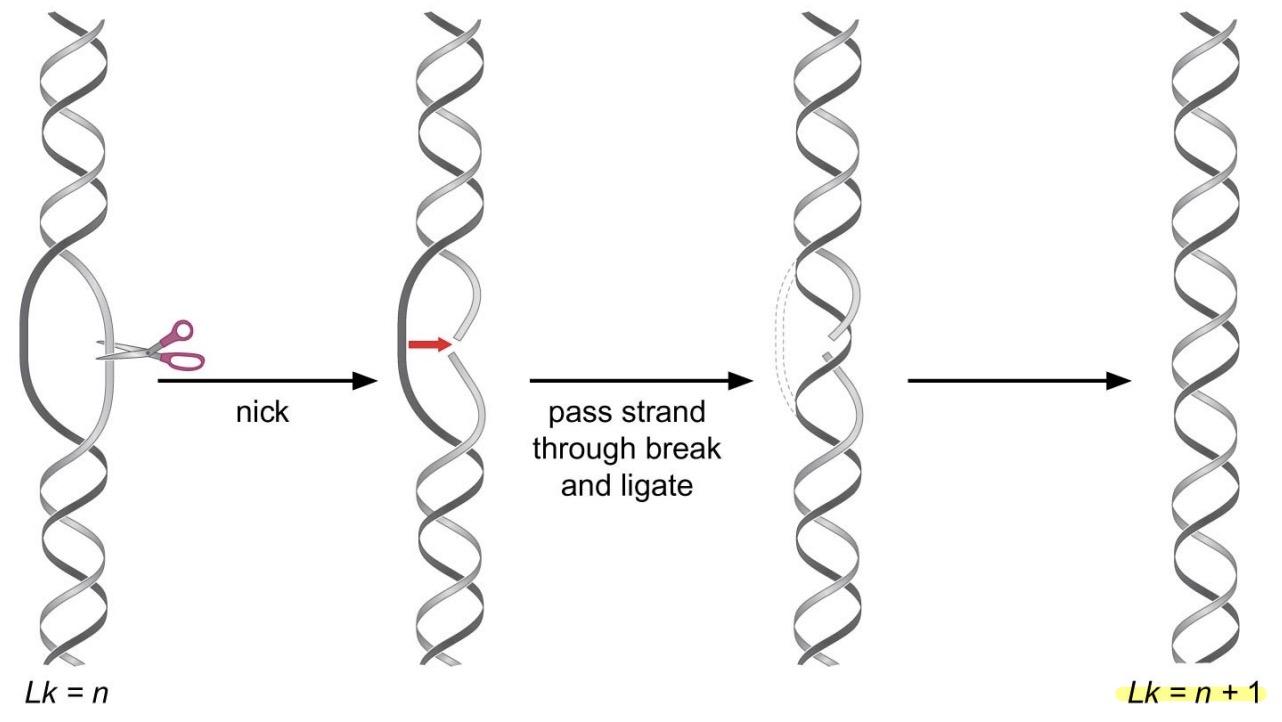
Type I (one turn is taken out)
1 step reaction: make transient single-strand break in DNA -> allow uncut stand to pass through break before resealing the nick
does not require ATP
Type II
2-step reaction: make transient double-strand break in DNA -> pass a segment of uncut duplex through DNA before resealing the break
requires ATP
E.g. DNA gyrase (prokaryotes)
introduces negative supercoiling -> facilitates unwinding
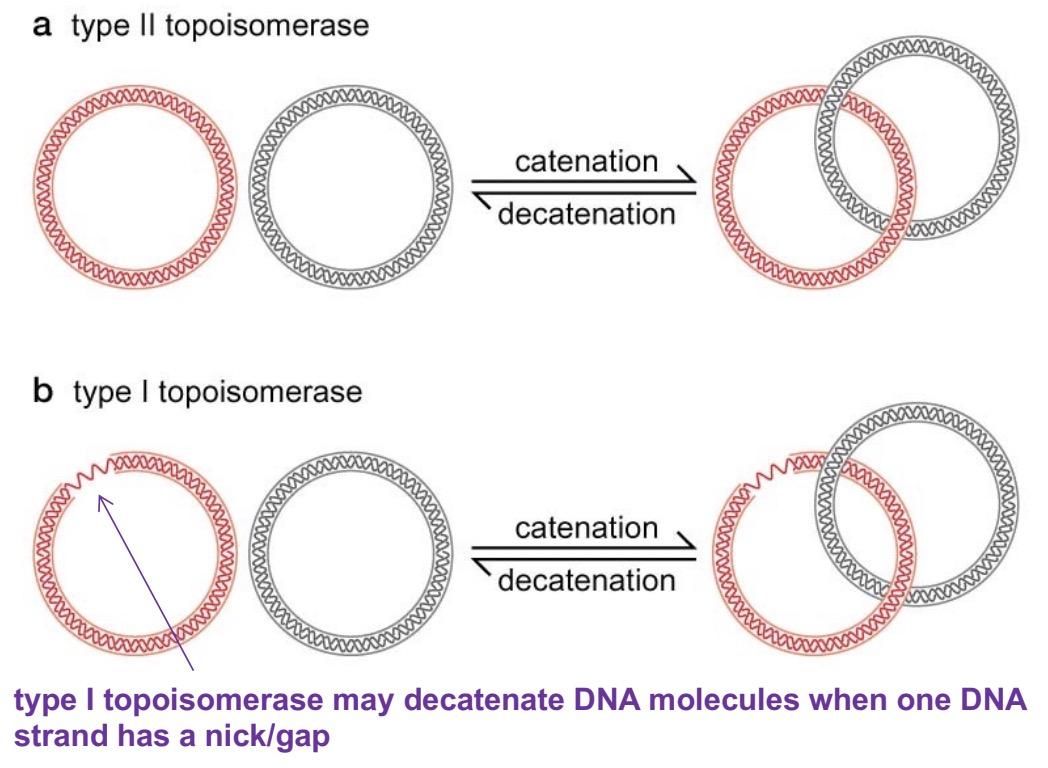
DNA catenation and decatenation
catenation: when circular DNA molecules are linked together like two rings of a chain
catenated DNA is commonly produced as a round of DNA replication is finished -> topoisomerases unlink DNA molecules (: decatenation)
requires type II topoisomerase
linear chromosomes:
DNA disentanglement by topoisomerase (usually type II) otherwise block separation of chromosomes
unknotting by topoisomerase (type II or type I if nick or gap)
Mechanism of DNA cleavage and rejoining by topoisomerases
tyrosine in active site of topoisomerase attacks phosphodiester bond in target DNA -> break in DNA -> topoisomerase is covalently joined to one of the broken ends via a phophotyrosine linkage (: conserves energy of phosphodiester bond) -> DNA can be resealed by reversing original reaction
Model for reaction cycle catalyzed by type I topoisomerase
topoisomerase binds to segment of duplex DNA where strands are melted. One strand binds in enzyme’s cleft that places it near active-site Tyr
strand is cleaved, generating covalent DNA-tyrosine intermediate (other end of DNA is also tightly bound to enzyme). Conformational change of enzyme opens gap in cleaved strand
second strand passes through gap and binds to DNA-binding site in ‘donut-shaped’ hole in protein
Conformational change brings cleaved DNA ends back together (OH end attacks phosphotyrosine bond)
enzyme opens up and releases DNA (DNA: Lk +1)
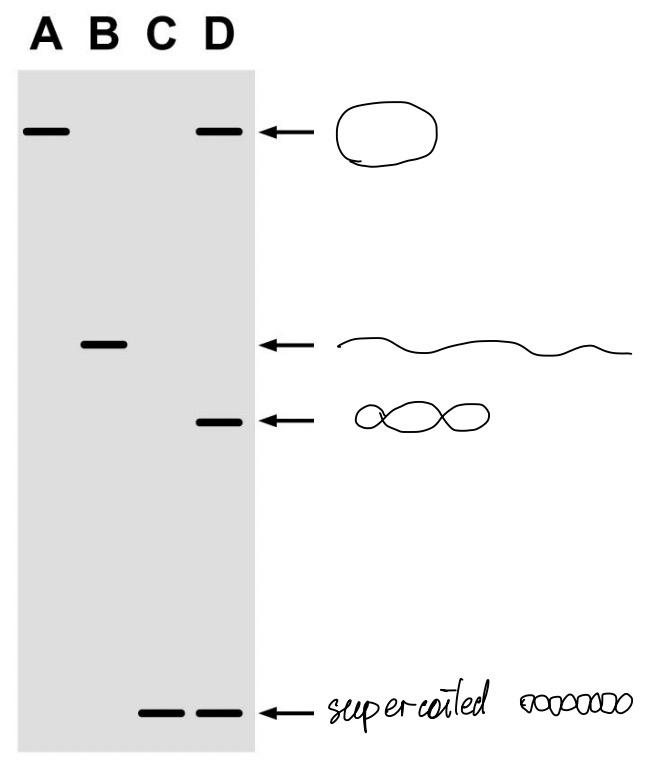
DNA topoisomers can be separated by electrophoresis
DNA topoisomers (same length, different linking numbers)
Ethidium ions cause DNA to unwind
a large, flat, multiringed cation
intercalate between stacked base pairs
unwind by 26 degree (36.5 bp/turn)
Summary
DNA is usually in the form of a right-handed double helix. The helix consists of two polydeoxynucleotide chains. Each chain is an alternating polymer of deoxyribose sugars and phosphates that are joined together via phosphodiester linkages. One of four bases protrudes from each sugar: adenine and guanine, which are purines, and thymine and cytosine, which are pyrimidines. Although the sugar-phosphate backbone is regular, the order of bases is irregular, and this is responsible for the information content of DNA. Each chain has a 5' to 3' polarity, and the two chains of the double helix are oriented in an antiparallel manner- that is, they run in opposite directions.
The polynucleotide chains are held together by base pairing and base stacking. Pairing is mediated by hydrogen bonds and results in the release of water molecules, increasing entropy. Base stacking also contributes to the stability of the double helix by favorable electron cloud interactions between the bases (van der Waals forces) and by burying the hydrophobic surfaces of the bases (the hydrophobic effect).
Hydrogen bonding is specific: Adenine on one chain is paired with thymine on the other chain, whereas guanine is paired with cytosine. This strict base pairing reflects the fixed locations of hydrogen atoms in the purine and pyrimidine bases in the forms of those bases found in DNA. Adenine and cytosine almost always exist in the amino as opposed to the imino-tautomeric form, whereas guanine and thymine almost always exist in the keto as opposed to the enol form. The complementarity between the bases on the two strands gives DNA its self-coding character.
The two strands of the double helix fall apart (denature) upon exposure to high temperature, extremes of pH, or any agent that causes the breakage of hydrogen bonds. Following slow return to normal cellular conditions, the denatured single strands can specifically reassociate to biologically active double helices (renature or anneal).
DNA in solution has a helical periodicity of ~ 10.5 bp per turn of the helix. The stacking of base pairs upon each other creates a helix with two grooves. Because the sugars protrude from the bases at an angle of ~ 120°, the grooves are unequal in size. The edges of each base pair are exposed in the grooves, creating a pattern of hydrogen-bond donors and acceptors and of hydrophobic groups that identify the base pair. The wider (or major) groove is richer in chemical information than the narrow (or minor) groove and is more important for recognition by nucleotide sequence-specific binding proteins.
Almost all cellular DNAs are extremely long molecules, with only one DNA molecule within a given chromosome.
Eukaryotic cells accommodate this extreme length in part by wrapping the DNA around protein particles known as nucleosomes. Most DNA molecules are linear, but some DNAs are circles, as is often the case for the chromosomes of prokaryotes and for certain viruses.
DNA is flexible. Unless the molecule is topologically constrained, it can freely rotate to accommodate changes in the number of times the two strands twist about each other.
DNA is topologically constrained when it is in the form of a covalently closed circle or when it is entrained in chromatin. The linking number is an invariant topological property of covalently closed, circular DNA. It is the number of times one strand would have to be passed through the other strand in order to separate the two circular strands. The linking number is the sum of two interconvertible geometric properties: twist, which is the number of times the two strands are wrapped around each other; and the writhing number, which is the number of times the long axis of the DNA crosses over itself in space. DNA is relaxed under physiological conditions when it has ~ 10.5 bp per turn and is free of writhe. If the linking number is decreased, then the DNA becomes torsionally stressed, and it is said to be negatively supercoiled. DNA in cells is usually negatively supercoiled by ~ 6%.
The left-handed wrapping of DNA around nucleosomes introduces negative supercoiling in eukaryotes. In prokaryotes, which lack histones, the enzyme DNA gyrase is responsible for generating negative supercoils. DNA gyrase is a member of the type II family of topoisomerases. These enzymes change the linking number of DNA in steps of two by making a transient break in the double helix and passing a region of duplex DNA through the break. Some type II topoisomerases relax supercoiled DNA, whereas DNA gyrase generates negative supercoils. Type I topoisomerases also relax supercoiled DNAs but do so in steps of one in which one DNA strand is passed through a transient nick in the other strand.