Chemical Kinetics
What is Chemical Kinetics?
Chemical kinetics, also called reaction kinetics, helps us understand the rates of reactions and how it is influenced by certain conditions.
It further helps to gather and analyse information about the mechanism of the reaction and define the characteristics of a chemical reaction.
Rate of Formations and Disappearances:
In any chemical reaction, as the reaction proceeds, the amount of reactants decreases, whereas the amount of products increases.
One has to understand that the rate of the overall reaction depends on the rate at which reactants are consumed or the rate at which the products are formed.
If a graph is plotted between the concentration of reactants and products and time, the rate of formation of products and the rate of disappearance of reactants can be easily calculated from the slope of curves for products and reactants.
The overall rate of the reaction may or may not be equal to the rate of formations and disappearances.
Product concentration is zero at time t = 0
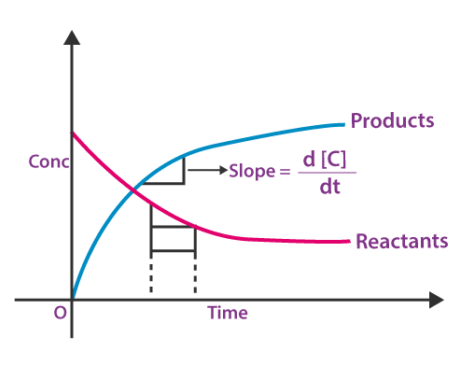
at time t = 0, both reactants and products are present.
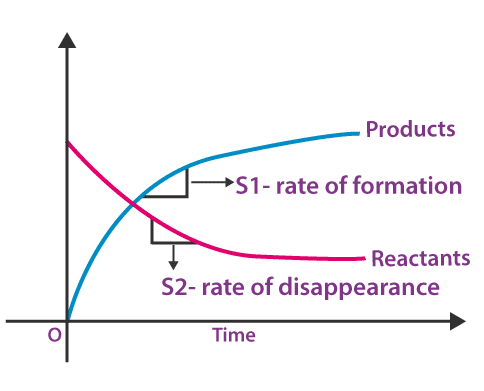
From the graph, it is understood that the slope of the reactants curve is negative and that for product curve is positive, indicating the concentration of reactants and products decreases and increases, respectively.
We will take a simple reaction as an example to illustrate how the rate of overall reactions, rate of disappearances of reactants and rate of formation of products are related.
Let us take the reaction of the formation of water.
2H2 + O2 → 2H2O
From the balanced equation, it is under that for one mole of O2 consumed, 2 moles of H2 will be consumed, and 2 moles of H2O will be formed. Say, the reaction proceeds for 10 mins, taking 1 mole of H2 and O2 each in the reaction mixture.
2H2 + O2 → 2H2O
t = 0 1 1 0
t = 10 mins 1 – 0.5 1 – 0.25 0.5
Say after 10 minutes, 0.5 moles of H2 is consumed, and according to stoichiometry, 0.25 moles of O2 is consumed, and 0.5 moles of H2O is formed. Now, let us calculate the rates for H2, O2 and H2O for the first 10 minutes.
Rate of disappearance of H2:
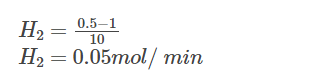
Rate of disappearance of O2:
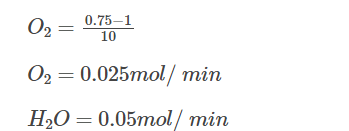
Rate of formation of H2O:
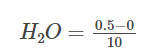
From the above calculations, we can see that rate at which H2 is consumed is twice the rate at which O2 is consumed.
So, the stoichiometry of the reaction relates rates of formation and disappearances of different reactants and products as follows:

Average and Instantaneous Rate:
The rate of reaction can be classified into average and instantaneous rates depending on the amount of time period. If the time period taken is finite, then it’s called the average rate and is represented as,
𝑟avg=Δ[𝐶]Δ𝑡
Δl → change in concentration
Δt → change in time
ravg → average rate
The average rate doesn’t give exact information in most cases about the completion of the reaction.
For example, let us consider the hydrolysis of esters to form acid and alcohol.
𝑅𝐶𝑂𝑂𝑅′→𝐻2𝑂𝑅𝐶𝑂𝑂𝐻+𝑅′𝑂𝐻
Say, at time t = 0, and there was 1 M solution of ester which becomes 0.5 M in 30 mins.
So, it is logical for us to assume that the reaction will be 100% completed in 1 hour.
But in reality, the reaction takes more than 3 hours to reach completion.
So, to get a broader insight into the time taken for completion and other purposes, “Instantaneous rate is used, which is represented as,
𝑟inst=limΔ𝑡→0Δ[𝐶]Δ𝑡=𝑑[𝐶]𝑑𝑡
From above, it’s understood that the time period taken is almost zero, from t = 0 to t = 0.0000 …1 second.
This eventually comes out to be the differential of change in concentration with respect to time.
For all practical purposes, the instantaneous rate is used, which can be calculated from the concentration in the time graph by finding a tangent at a point.

The unit of rate is Moll-1 s-1 because it is concentration/time, and concentration is expressed in terms of Molarity/mol l-1).
It can also be Nm-2/s if the active mass is used in terms of partial pressures.
Depending on other units of time, it can also be mol l-1 min-1 or mol l-1 hour-1 etc.
Factors Affecting the Reaction Rate:
The rate of a reaction can be altered if any of the following parameters are changed.
Concentration of Reactants:
According to collision theory, reactant molecules collide with each other to form products.
If the concentration of reactants is increased, the number of colliding particles will increase, thereby increasing the rate of reaction.
Nature of the Reactants:
The reaction rate also depends on the types of substances that are reacting. If we consider acid/base reactions, salt formation and ion exchange, they are mostly fast reactions.
During the formation of a covalent bond between the molecules that results in the formation of larger molecules, the reaction that takes place is usually slower.
Furthermore, the nature and strength of bonds in reactant molecules significantly affect the rate of their transformation into products.
Physical State of Reactants:
The physical state of a reactant, whether it is solid, liquid or gas, can greatly affect the rate of change.
To discuss it further, if reactants are in the same phase, let’s say they are in an aqueous solution, here the thermal motion will bring them together.
If they are in different phases, then the reaction will be limited to the interface between the reactants.
The reaction mainly occurs only at their area of contact, in the case of a liquid and a gas, at the surface of the liquid.
Surface Area of Reactants:
If we take two solids, the particles that are at the surface will take part in the reaction.
Likewise, if we want to crush a solid into smaller parts, more particles will be present at the surface.
What it means is that the frequency of collisions between these and reactant particles will most likely increase.
As a result, the reaction will occur more rapidly.
When two or more reactants are in the same phase of fluid, their particles collide more often than when either or both are in the solid phase or when they are in a heterogeneous mixture. In a heterogeneous medium, the collision between the particles occurs at an interface between phases.
Compared to the homogeneous case, the number of collisions between reactants per unit time is significantly reduced, and so is the reaction rate.
Temperature:
If the temperature is increased, the number of collisions between reactant molecules per second (frequency of collision) increases, thereby increasing the rate of the reaction.
But depending on whether the reaction is endothermic or exothermic, an increase in temperature increases the rate of forward or backward reactions, respectively.
In a system where more than one reaction is possible, the same reactants can produce different products under different temperature conditions.
At 100 0C in the presence of dilute sulphuric acid, diethyl ether is formed from ethanol.
2CH3CH2OH → CH3CH2OCH2CH3+H2O
At 180 0C in the presence of dilute sulphuric acid, ethylene is the major product.
CH3CH2OH → C2H4+H2O
Effect of Solvent:
The nature of the solvent also depends on the reaction rate of the solute particles.
Example:
When sodium acetates react with methyl iodide, it gives methyl acetate and sodium iodide.
CH3CO2Na(sol)+CH3I(liq)→CH3CO2CH3(sol)+NaI(sol)
The above reaction occurs faster in organic solvents such as DMF (dimethylformamide) than in CH3OH (methanol) because methanol is able to form a hydrogen bond with CH3CO2 – but DMF is not possible.
Catalyst:
Catalysts alter the rate of the reaction by changing the reaction mechanism.
There are two types of catalysts, namely, promoters and poisons, which increase and decrease the rate of reactions, respectively.
Positive Catalysts:
Catalysts that increase the rate of a chemical reaction are positive catalysts.
It increases the rate of reaction by lowering the activation energy barriers such that a large number of reaction molecules are converted into products, and thereby the percentage of yield of products increases.
Positive catalyst example:
In the preparation of NH3 by Haber’s process, iron oxide acts as a positive catalyst and increases the yield of ammonia in spite of less reaction of nitrogen.
Negative Catalysts:
Catalysts that decrease the rate of reaction are negative catalysts.
It decreases the rate of reaction by increasing the activation energy barrier, which decreases the number of reactant molecules to transform into products, and hence the rate of reaction decreases.
Negative catalyst example:
The decomposition of hydrogen peroxide into water and oxygen is retarded by using acetanilide, and this acts as a negative catalyst to decrease the rate of decomposition of hydrogen peroxide.
 Knowt
Knowt
