
Chapter 5: Microbial Metabolism
5.1: Catabolic and Anabolic Reactions
Define metabolism and describe the fundamental differences between anabolism and catabolism.
Metabolism: The sum of all chemical reactions in living organisms. Two Classes of metabolism:
Catabolism: the reaction that breaks down chemicals and releases energy. They are called catabolic or degradative reactions. Catabolic reactions are hydrolytic, use water to break bonds, and exergonic, produce more energy than consumed. They help provide building block for anabolic reactions.
Anabolism: the reaction that builds complex chemicals and uses energy. They are called anabolic or biosynthetic reactions. Anabolic processes involve dehydration synthesis, release water, and are endergonic, consume more energy than produced.
Identify the role of ATP as an intermediate between catabolism and anabolism.
ATP is an intermediate between catabolism and anabolism because ATP catabolic reactions build ATP molecules and anabolic reactions breakdown reactions to produce energy. ATP consists of an adenine, a ribose, and three phosphate groups.
ATP → ADP + 🅟i + energy
ADP + 🅟i + energy → ATP
The breakdown of glucose is a catabolic reaction to bind ADP and the free phosphate to form ATP and have byproducts of CO₂ and H₂O. ATP breakdown is an anabolic reaction to help build macromolecules with the byproduct of ADP and the free phosphate.
Metabolic pathways are determined by enzymes.
5.2: Enzymes
Collision Theory: the principle that chemical reaction occur because energy is gained as particles collide.
Collision causing chemical reactions is determined by:
velocities of colliding particles; up to a point, high velocity means more probable collision.
their energy
Their specific chemical configuration
Activation energy: amount of energy needed to disrupt the stable electronic configuration of any specific molecule so that the electrons can be rearranged.
Reaction Rate: frequency of collisions. Raise rate by raising temperature.
Identify the components of an enzyme.
Components of enzymes:
Enzymes are catalysts. Each acts on a specific substrate(s).
Some enzymes consist of only proteins. Other have a protein portion (apoenzyme), which is inactive by itself
They also have a nonprotein portion (cofactor), which can be ions of iron, zinc, magnesium, calcium, etc. Cofactor that is an organic molecule is a coenzyme. Cofactors can form bridge for enzyme and substrate.
Coenzymes can accept or donate atoms to substrate, and some can accept electrons to donate in subsequent reactions.
Most important coenzymes are nicotinamide adenine dinucleotide (NAD⁺) and nicotinamide adenine dinucleotide phosphate (NADP⁺). Both contain derivatives of niacin.
Flavin enzymes like flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) contain derivatives of riboflavin and are electron carriers
Coenzyme A (CoA) contains derivative of pantothenic acid and is important to synthesis and breakdown of fats and Krebs cycle.
Holoenzyme: an apoenzyme and cofactor joined together.
Describe the mechanism of enzymatic action.
Optimal conditions can result in catalyzation reaction rates of 108 -1010 times higher than without an enzyme. The turnover number (maximum number of reactions per second) is between 1 and 10,000 and can even reach 500,000.
Enzymatic action:
Surface of the substrate contacts a specific area of enzyme, called the active site.
Temporary intermediate compound forms (enzyme-substrate complex).
Substrate molecule is transformed by atomic rearrangement, substrate breakdown, or a combination with another substrate.
Transformed substrates are released because they no longer fit.
Unchanged enzyme can react with more substrates.
List the factors that influence enzymatic activity.
Factors that influence enzymatic activity:
Temperature: Higher temperature means more reactions, but too high temperature drops reaction rate. Best temperature for disease-causing bacteria is 35-40°C. Decline in reaction rate with out-of-range temperature is due to enzyme denaturation. Denaturation changes arrangement of amino acids in the active site. Denaturation can be partially or completely reversed to a certain point. Can also be denatured by concentrated acids, bases, heavy-metal ions, alcohol, and UV radiation.
pH: Enzymes have optimal pH range. Acids and Bases alter the protein structure because the H⁺ or OH⁻ compete with hydrogen and ionic bonds in enzyme.
Substrate Concentration: Enzyme is in saturation when the substrate concentration is really high. Adding more substrates will not affect rate if it is saturation.
Inhibitors: Bind to the active site to prevent reactions. Cyanide, arsenic, and mercury combine with enzymes to prevent functioning. Feedback inhibition, or end-product inhibition, stops a cell from producing more substances than needed. Some metabolic reactions require several steps of synthesis to produce the end-product that will allosterically inhibit activity.
Distinguish competitive and noncompetitive inhibition.
Competitive vs noncompetitive inhibition:
Competitive inhibitors actively compete with normal substrates for the active site. Some bind irreversibly to amino acids. Some bind temporarily to slow reaction rates. Increasing substrate concentration to overcome competition. One type of competitive inhibitor is sulfanilamide (antibacterial drug) which competes against para-aminobenzoic acid (PABA), preventing the synthesis of folic acid, preventing bacterial growth.
Noncompetitive inhibitors interact with another part of the enzyme, not the active site. This is allosteric inhibition and it involves contact with the allosteric site. Some enzymes require metal ions to activate and these inhibitors can bind/tie these metal ion activators. Cyanide binds iron in iron-containing enzymes and fluoride can bind calcium or magnesium. Substances like cyanide and fluoride are called enzymes poisons because of the permanence.
Escherichia coli demonstrates feedback inhibition in the synthesis of the amino acid isoleucine, crucial for cell growth. In its metabolic pathway, threonine is converted to isoleucine through five enzymatic steps, with isoleucine accumulation inhibiting the first enzyme of the pathway, halting further synthesis. This feedback mechanism regulates not only isoleucine production but also the synthesis of other amino acids, vitamins, purines, and pyrimidines.
Define ribozyme.
Ribozyme: A unique type of RNA the cut and splice RNA as well as involve themselves in protein synthesis at ribosomes.
5.3: Energy Production
Explain the term oxidation-reduction.
Oxidation is the removal of electrons, producing energy.
Oxidation-reduction: Also known as a redox reaction, when one molecule gains electrons (reduced) from another molecule losing electrons (oxidized).
Most biological oxidations involve losing hydrogen atoms, they are called dehydrogenation reactions. NAD⁺ is reduced by accepting two hydrogen atoms, accepting two electrons and one proton, forming NADH, which can help in the production of ATP.
List and provide examples of three types of phosphorylation reactions that generate ATP.
Phosphorylation: the addition of a phosphate to a chemical compound.
Three mechanisms for ATP-producing Phosphorylation reactions:
Substrate-Level Phosphorylation: A high-energy phosphate is directly transferred from phosphorylated compound to ADP. Phosphate gained energy in earlier reaction when substrate was oxidized.
C-C-C⁓ Phosphate + ADP → C-C-C + ATP
Oxidative phosphorylation: electrons are transferred from organic compounds to electron carriers, primarily NAD⁺ and FAD. These electrons move through a series of carriers known as the electron transport chain, ultimately reaching oxygen or other oxidized molecules, releasing energy in the process. This energy is partially used to synthesize ATP from ADP via chemiosmosis in the plasma membrane of prokaryotes and the inner mitochondrial membrane of eukaryotes.
Photophosphorylation: Only in photosynthetic cells. Converts light energy to chemical energy of ATP and NADPH which will synthesize organic molecules. Electron transport chain involved.
Explain the overall function of metabolic pathways.
Metabolic pathways are needed to control reactions and prevent large amounts of heat to be generated, damaging the cell.
5.4 Carbohydrate Catabolism
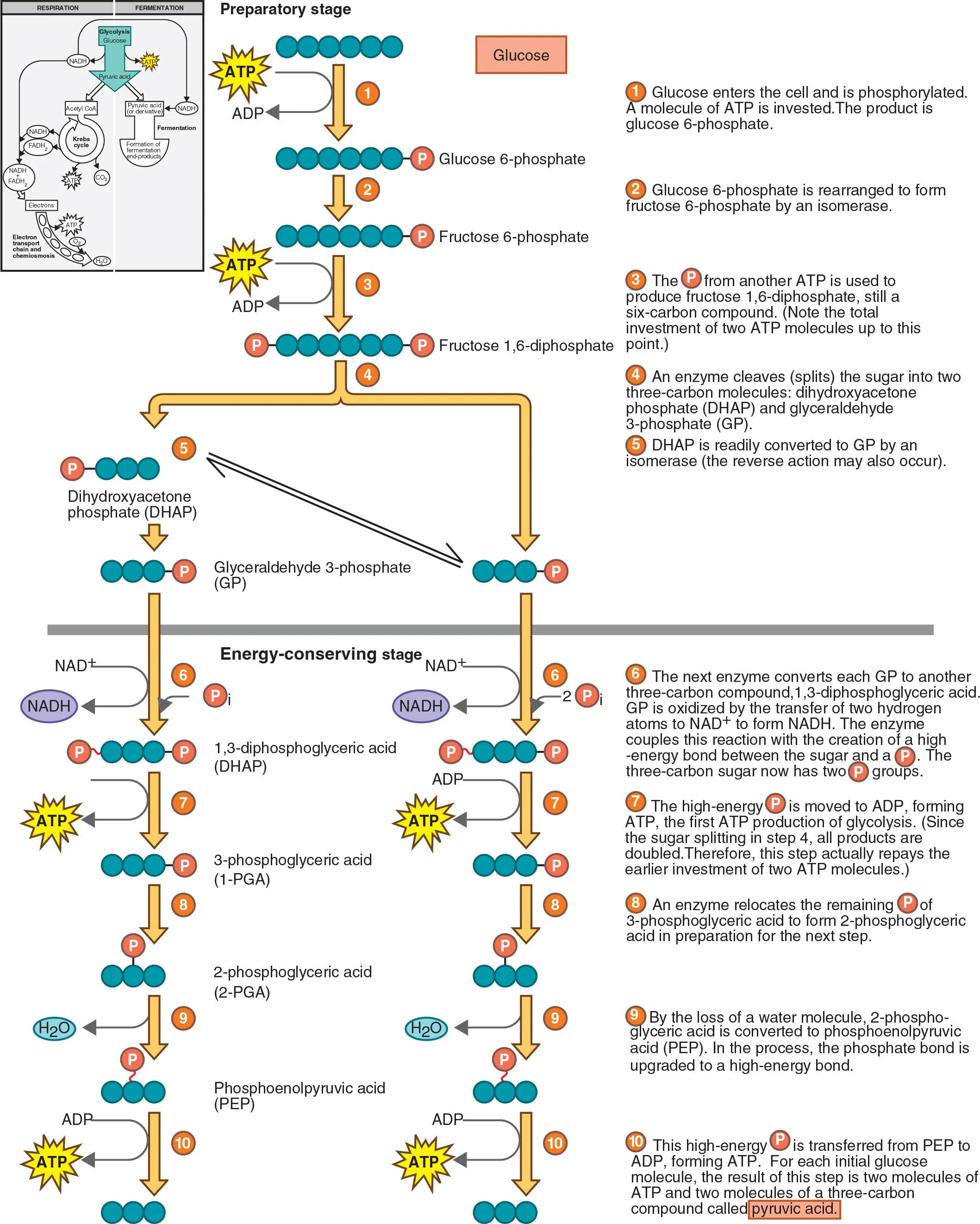

Describe the chemical reactions of glycolysis.
Cellular respiration and fermentation both start with glycolysis, also called the Embden-Meyerhof pathway. It does not require oxygen. The reaction is:
In the preparatory stage of glycolysis, two ATP molecules are used to phosphorylate and restructure a six-carbon glucose molecule, which is then split into two three-carbon compounds: glyceraldehyde (GP) and dihydroxyacetone phosphate (DHAP). DHAP can be readily converted into GP, leading to two molecules of GP being introduced into the subsequent reactions of glycolysis.
In the energy-conserving stage of glycolysis, the two three-carbon molecules are oxidized to two molecules of pyruvic acid. During this process, two molecules of NAD⁺ are reduced to NADH, and four molecules of ATP are formed through substrate-level phosphorylation. Net gain of two ATP molecules
Identify the functions of the pentose phosphate and Entner-Doudoroff pathways.
Pentose phosphate and Entner-Doudoroff pathways are alternatives to glycolysis for bacteria:
Pentose Phosphate Pathway: works with glycolysis to breakdown pentoses (5-carbon sugars) and glucose. Produces important intermediate pentoses for the synthesis of nucleic acids, glucose from carbon dioxide in photosynthesis, and certain amino acids. Important producer of reduced coenzyme NADPH. Net gain of one ATP. Bacteria that use it include Bacillus subtilis, E. coli, Leuconostoc mesenteroides, and Enteroccus faecalis. Also has this pathway in humans.
Entner-Doudoroff Pathway: Produces one NADPH, NADH, and ATP. Bacteria with specific enzyme can metabolize glucose without glycolysis or pentose phosphate pathway. Found in some gram-negative like Rhizobium, Pseudomonas, Agrobacterium, and cyanobacteria. Not really in gram-positive, but occurs in archaea, algae, and plants.
Explain the products of the Krebs cycle.
Krebs cycle also known as the tricarboxylic acid cycle or citric acid cycle.
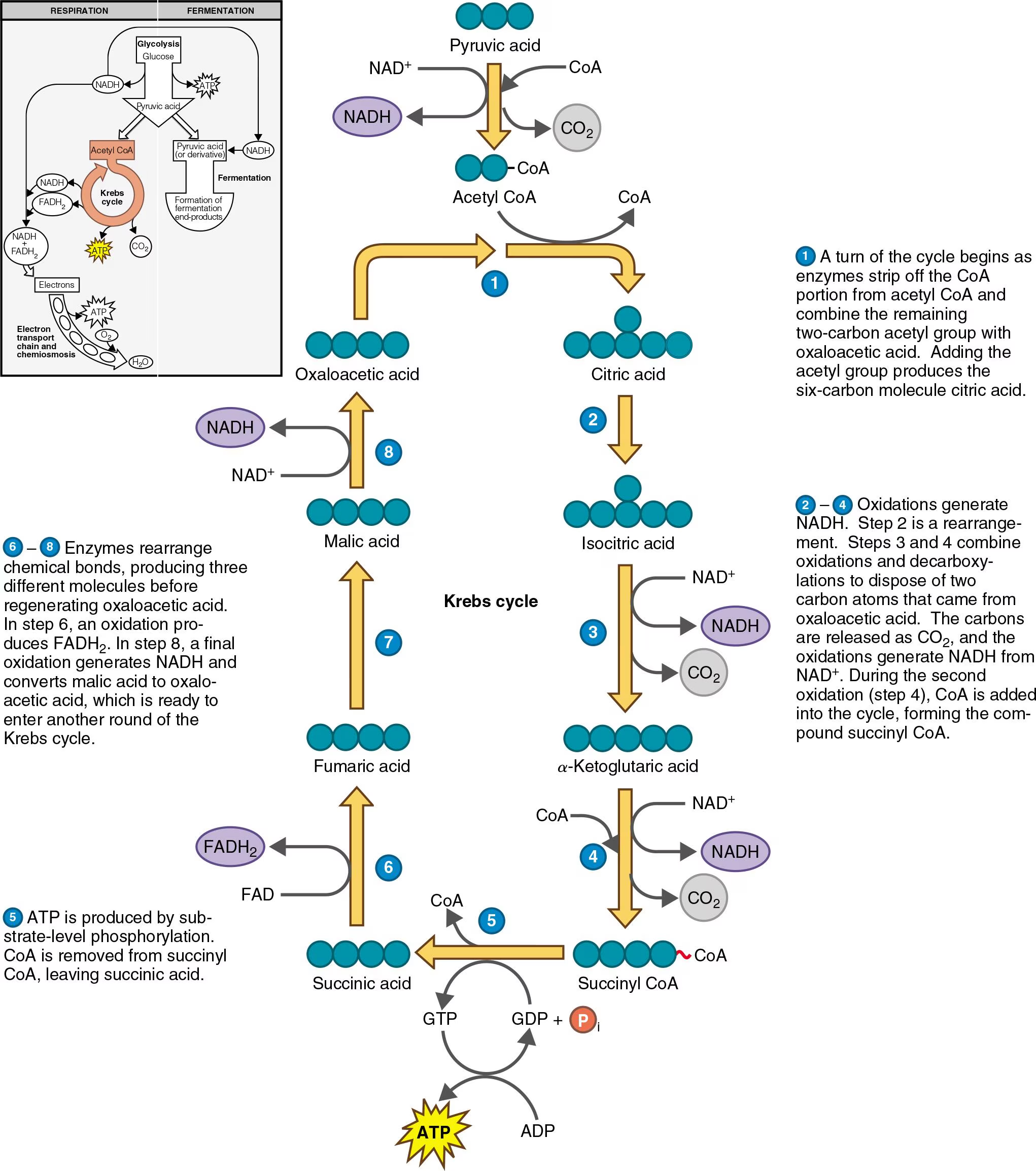
Acetyl-CoA Formation: Before entering the Krebs cycle, pyruvate from glycolysis is converted into acetyl-CoA by losing a carboxyl (-COOH) group (decarboxylation), releasing one CO₂ and forming the two-carbon acetyl group. This attaches to coenzyme A to form high energy bond and form acetyl coenzyme A. Pyruvic acid is oxidized, and NAD⁺ is reduced to NADH.
This is with 2 pyruvic acid, so two CO₂ are released, two NADH are produced, and two acetyl-CoA are formed.
(Step 1) Citrate Formation: Acetyl-CoA combines with four-carbon compound called oxaloacetate to form citrate. Requiring energy, it uses the high-energy bond between acetyl group and CoA.
Isomerization: Citrate is rearranged into isocitrate.
(Step 3) Oxidative Decarboxylation: Isocitrate is oxidized to alpha-ketoglutarate, producing NADH and releasing CO₂.
(Step 4) Further Decarboxylation: Alpha-ketoglutarate is further oxidized to succinyl-CoA, again producing NADH and CO₂. Hydrogen atoms released and picked up by coenzymes NAD⁺ and FAD. NAD⁺ picks up two electrons and one proton, reducing to NADH; FAD accepts two hydrogen atoms to be reduced to NADH₂
Now, all three carbon atoms in pyruvic acid are released as CO₂. Conversion of all six carbon atoms to CO₂ is completed in two turns of Krebs cycle.
Substrate-level Phosphorylation: Succinyl-CoA is converted to succinate, generating ATP (or GTP). Guanosine Triphosphate serves as an intermediary at this point.
Oxidation: Succinate is oxidized to fumarate, producing FADH₂.
Hydration: Fumarate is hydrated to malate.
Final Oxidation: Malate is oxidized to regenerate oxaloacetate, producing NADH. This oxaloacetate can then combine with another acetyl-CoA to repeat the cycle.
Products: The Krebs cycle produces 6 NADH, 2 FADH₂, 2 ATP (or GTP), and 4 CO₂ molecules. These electron carriers (NADH and FADH₂) are crucial for the electron transport chain, where most ATP is produced during aerobic respiration.
Describe the chemiosmotic mechanism of ATP generation.
Electron Transport Chain System: a sequence of carrier molecules that are capable of oxidation and reduction. As electrons pass through the chain, there is a release of energy to drive the chemiosmotic generation of ATP, making the final oxidation irreversible. Eukaryotes have it in the inner mitochondrial membrane and prokaryotes have it in the plasma membrane. Three classes of carrier models in electron transport chains:
Flavoproteins: contain flavin and are capable of performing oxidations and reductions, one important one being flavin mononucleotide (FMN). Can transfer both protons and electrons.
Ubiquinones (Coenzyme Q): small nonprotein carriers. Can transfer protons and electrons.
Cytochromes: Proteins with an iron-containing protein (-heme). The iron can be reduced (Fe²⁺) or oxidized (Fe³⁺). They can only transfer electrons Cytochromes in this chain include:
cytochrome b (cyt b)
cytochrome c₁ (cyt c₁)
cytochrome c (cyt c)
cytochrome a (cyt a)
cytochrome a₃ (cyt a₃)
In the electron transport chain system:
Electrons are transferred from NADH to FMN, the first carrier. Hydrogen atom with 2 electrons passes to FMN which picks up another hydrogen ion in the aqueous solution. NADH is oxidized to NAD⁺ and FMN is reduced to FMNH₂.
FMNH₂ passes 2H⁺ to other side of mitochondrial membrane and two electrons to Q. FMNH₂ is oxidized to FMN and Q picks up an addition 2H⁺ from surrounding membrane and releases it to other side of membrane.
Electrons are passed successively to cyt b, cyt c₁, cyt c, cyt a, and cyt a₃. Each cytochrome is reduced and oxidized. The last cytochrome passes the electrons to oxygen, which becomes negatively charged before picking up protons to form water. As you progress along the chain, each electron carrier has greater electronegativity before it. Since they can only transfer electrons, the buildup of protons provides enough energy for chemiosmotic mechanism for ATP generation.
Chemiosmosis mechanism: it involves oxidative phosphorylation. Energy released as a substance moves along a concentration gradient is used to generate ATP. The steps are:
As energetic electrons from NADPH pass down transport chain, some carriers (proton pumps) actively transport protons across membrane.Q transports electrons between first and second complexes, and cyt c transports them between second and third complexes.
Phospholipid membrane is impermeable to protons, so it establishes a gradient. The excess H⁺ on one side makes it more positively charged than another. The electrochemical gradient has potential energy, called proton motive force.
Protons on higher concentration side can only diffuse across membrane through protein channels that contain ATP synthase. Energy is released to synthesize ADP into ATP as protons flow.
Compare and contrast aerobic and anaerobic respiration.
Anerobic respiration: the final receptor is an inorganic molecule other than oxygen, rarely an organic molecule. Some bacteria, Pseudomonas and Bacillus, can use nitrate ion (NO3-) as final receptor to become nitrite ion (NO2-), nitrous oxide, or nitrogen gas. Other bacteria, Desulfovibrio, use sulfate (SO42-). Some archaea use CO2 to form methane. Sulfur and Nitrogen use are essential to nitrogen and sulfur cycles. Amount of ATP is varies, but never reaches as high as aerobic respiration.
Aerobic respiration: The final electron receptor is oxygen. The various transfers in the electron transport chain generate about 34 molecules of ATP from each glucose oxidized, three from each of ten molecules of NADH (total of 30) and two from each of the two FADH₂ (total of four). Aerobic respiration in prokaryotes generates 38 molecules of ATP: 34 from chemiosmosis plus four generated by oxidation in glycolysis and Krebs cycle. Eukaryotes produce two fewer ATPs because NADH cannot enter mitochondria.
Describe the chemical reactions of, and list some products of, fermentation.
Fermentation can convert pyruvic acid into an organic product, having NAD⁺ and NADP⁺ regenerated to enter another round of glycolysis. Fermentation
5.5
Describe how lipids and proteins undergo catabolism.
Provide two examples of the use of biochemical tests to identify bacteria in the laboratory.
Compare and contrast cyclic and noncyclic photophosphorylation.
Compare and contrast the light-dependent and lightindependent reactions of photosynthesis.
Compare and contrast oxidative phosphorylation and photophosphorylation.
Write a sentence to summarize energy production in cells.
Categorize the various nutritional patterns among organisms according to carbon source and mechanisms of carbohydrate catabolism and ATP generation.
Describe the major types of anabolism and their relationship to catabolism.
Define amphibolic pathways.
Chapter 5: Microbial Metabolism
5.1: Catabolic and Anabolic Reactions
Define metabolism and describe the fundamental differences between anabolism and catabolism.
Metabolism: The sum of all chemical reactions in living organisms. Two Classes of metabolism:
Catabolism: the reaction that breaks down chemicals and releases energy. They are called catabolic or degradative reactions. Catabolic reactions are hydrolytic, use water to break bonds, and exergonic, produce more energy than consumed. They help provide building block for anabolic reactions.
Anabolism: the reaction that builds complex chemicals and uses energy. They are called anabolic or biosynthetic reactions. Anabolic processes involve dehydration synthesis, release water, and are endergonic, consume more energy than produced.
Identify the role of ATP as an intermediate between catabolism and anabolism.
ATP is an intermediate between catabolism and anabolism because ATP catabolic reactions build ATP molecules and anabolic reactions breakdown reactions to produce energy. ATP consists of an adenine, a ribose, and three phosphate groups.
ATP → ADP + 🅟i + energy
ADP + 🅟i + energy → ATP
The breakdown of glucose is a catabolic reaction to bind ADP and the free phosphate to form ATP and have byproducts of CO₂ and H₂O. ATP breakdown is an anabolic reaction to help build macromolecules with the byproduct of ADP and the free phosphate.
Metabolic pathways are determined by enzymes.
5.2: Enzymes
Collision Theory: the principle that chemical reaction occur because energy is gained as particles collide.
Collision causing chemical reactions is determined by:
velocities of colliding particles; up to a point, high velocity means more probable collision.
their energy
Their specific chemical configuration
Activation energy: amount of energy needed to disrupt the stable electronic configuration of any specific molecule so that the electrons can be rearranged.
Reaction Rate: frequency of collisions. Raise rate by raising temperature.
Identify the components of an enzyme.
Components of enzymes:
Enzymes are catalysts. Each acts on a specific substrate(s).
Some enzymes consist of only proteins. Other have a protein portion (apoenzyme), which is inactive by itself
They also have a nonprotein portion (cofactor), which can be ions of iron, zinc, magnesium, calcium, etc. Cofactor that is an organic molecule is a coenzyme. Cofactors can form bridge for enzyme and substrate.
Coenzymes can accept or donate atoms to substrate, and some can accept electrons to donate in subsequent reactions.
Most important coenzymes are nicotinamide adenine dinucleotide (NAD⁺) and nicotinamide adenine dinucleotide phosphate (NADP⁺). Both contain derivatives of niacin.
Flavin enzymes like flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) contain derivatives of riboflavin and are electron carriers
Coenzyme A (CoA) contains derivative of pantothenic acid and is important to synthesis and breakdown of fats and Krebs cycle.
Holoenzyme: an apoenzyme and cofactor joined together.
Describe the mechanism of enzymatic action.
Optimal conditions can result in catalyzation reaction rates of 108 -1010 times higher than without an enzyme. The turnover number (maximum number of reactions per second) is between 1 and 10,000 and can even reach 500,000.
Enzymatic action:
Surface of the substrate contacts a specific area of enzyme, called the active site.
Temporary intermediate compound forms (enzyme-substrate complex).
Substrate molecule is transformed by atomic rearrangement, substrate breakdown, or a combination with another substrate.
Transformed substrates are released because they no longer fit.
Unchanged enzyme can react with more substrates.
List the factors that influence enzymatic activity.
Factors that influence enzymatic activity:
Temperature: Higher temperature means more reactions, but too high temperature drops reaction rate. Best temperature for disease-causing bacteria is 35-40°C. Decline in reaction rate with out-of-range temperature is due to enzyme denaturation. Denaturation changes arrangement of amino acids in the active site. Denaturation can be partially or completely reversed to a certain point. Can also be denatured by concentrated acids, bases, heavy-metal ions, alcohol, and UV radiation.
pH: Enzymes have optimal pH range. Acids and Bases alter the protein structure because the H⁺ or OH⁻ compete with hydrogen and ionic bonds in enzyme.
Substrate Concentration: Enzyme is in saturation when the substrate concentration is really high. Adding more substrates will not affect rate if it is saturation.
Inhibitors: Bind to the active site to prevent reactions. Cyanide, arsenic, and mercury combine with enzymes to prevent functioning. Feedback inhibition, or end-product inhibition, stops a cell from producing more substances than needed. Some metabolic reactions require several steps of synthesis to produce the end-product that will allosterically inhibit activity.
Distinguish competitive and noncompetitive inhibition.
Competitive vs noncompetitive inhibition:
Competitive inhibitors actively compete with normal substrates for the active site. Some bind irreversibly to amino acids. Some bind temporarily to slow reaction rates. Increasing substrate concentration to overcome competition. One type of competitive inhibitor is sulfanilamide (antibacterial drug) which competes against para-aminobenzoic acid (PABA), preventing the synthesis of folic acid, preventing bacterial growth.
Noncompetitive inhibitors interact with another part of the enzyme, not the active site. This is allosteric inhibition and it involves contact with the allosteric site. Some enzymes require metal ions to activate and these inhibitors can bind/tie these metal ion activators. Cyanide binds iron in iron-containing enzymes and fluoride can bind calcium or magnesium. Substances like cyanide and fluoride are called enzymes poisons because of the permanence.
Escherichia coli demonstrates feedback inhibition in the synthesis of the amino acid isoleucine, crucial for cell growth. In its metabolic pathway, threonine is converted to isoleucine through five enzymatic steps, with isoleucine accumulation inhibiting the first enzyme of the pathway, halting further synthesis. This feedback mechanism regulates not only isoleucine production but also the synthesis of other amino acids, vitamins, purines, and pyrimidines.
Define ribozyme.
Ribozyme: A unique type of RNA the cut and splice RNA as well as involve themselves in protein synthesis at ribosomes.
5.3: Energy Production
Explain the term oxidation-reduction.
Oxidation is the removal of electrons, producing energy.
Oxidation-reduction: Also known as a redox reaction, when one molecule gains electrons (reduced) from another molecule losing electrons (oxidized).
Most biological oxidations involve losing hydrogen atoms, they are called dehydrogenation reactions. NAD⁺ is reduced by accepting two hydrogen atoms, accepting two electrons and one proton, forming NADH, which can help in the production of ATP.
List and provide examples of three types of phosphorylation reactions that generate ATP.
Phosphorylation: the addition of a phosphate to a chemical compound.
Three mechanisms for ATP-producing Phosphorylation reactions:
Substrate-Level Phosphorylation: A high-energy phosphate is directly transferred from phosphorylated compound to ADP. Phosphate gained energy in earlier reaction when substrate was oxidized.
C-C-C⁓ Phosphate + ADP → C-C-C + ATP
Oxidative phosphorylation: electrons are transferred from organic compounds to electron carriers, primarily NAD⁺ and FAD. These electrons move through a series of carriers known as the electron transport chain, ultimately reaching oxygen or other oxidized molecules, releasing energy in the process. This energy is partially used to synthesize ATP from ADP via chemiosmosis in the plasma membrane of prokaryotes and the inner mitochondrial membrane of eukaryotes.
Photophosphorylation: Only in photosynthetic cells. Converts light energy to chemical energy of ATP and NADPH which will synthesize organic molecules. Electron transport chain involved.
Explain the overall function of metabolic pathways.
Metabolic pathways are needed to control reactions and prevent large amounts of heat to be generated, damaging the cell.
5.4 Carbohydrate Catabolism


Describe the chemical reactions of glycolysis.
Cellular respiration and fermentation both start with glycolysis, also called the Embden-Meyerhof pathway. It does not require oxygen. The reaction is:
In the preparatory stage of glycolysis, two ATP molecules are used to phosphorylate and restructure a six-carbon glucose molecule, which is then split into two three-carbon compounds: glyceraldehyde (GP) and dihydroxyacetone phosphate (DHAP). DHAP can be readily converted into GP, leading to two molecules of GP being introduced into the subsequent reactions of glycolysis.
In the energy-conserving stage of glycolysis, the two three-carbon molecules are oxidized to two molecules of pyruvic acid. During this process, two molecules of NAD⁺ are reduced to NADH, and four molecules of ATP are formed through substrate-level phosphorylation. Net gain of two ATP molecules
Identify the functions of the pentose phosphate and Entner-Doudoroff pathways.
Pentose phosphate and Entner-Doudoroff pathways are alternatives to glycolysis for bacteria:
Pentose Phosphate Pathway: works with glycolysis to breakdown pentoses (5-carbon sugars) and glucose. Produces important intermediate pentoses for the synthesis of nucleic acids, glucose from carbon dioxide in photosynthesis, and certain amino acids. Important producer of reduced coenzyme NADPH. Net gain of one ATP. Bacteria that use it include Bacillus subtilis, E. coli, Leuconostoc mesenteroides, and Enteroccus faecalis. Also has this pathway in humans.
Entner-Doudoroff Pathway: Produces one NADPH, NADH, and ATP. Bacteria with specific enzyme can metabolize glucose without glycolysis or pentose phosphate pathway. Found in some gram-negative like Rhizobium, Pseudomonas, Agrobacterium, and cyanobacteria. Not really in gram-positive, but occurs in archaea, algae, and plants.
Explain the products of the Krebs cycle.
Krebs cycle also known as the tricarboxylic acid cycle or citric acid cycle.

Acetyl-CoA Formation: Before entering the Krebs cycle, pyruvate from glycolysis is converted into acetyl-CoA by losing a carboxyl (-COOH) group (decarboxylation), releasing one CO₂ and forming the two-carbon acetyl group. This attaches to coenzyme A to form high energy bond and form acetyl coenzyme A. Pyruvic acid is oxidized, and NAD⁺ is reduced to NADH.
This is with 2 pyruvic acid, so two CO₂ are released, two NADH are produced, and two acetyl-CoA are formed.
(Step 1) Citrate Formation: Acetyl-CoA combines with four-carbon compound called oxaloacetate to form citrate. Requiring energy, it uses the high-energy bond between acetyl group and CoA.
Isomerization: Citrate is rearranged into isocitrate.
(Step 3) Oxidative Decarboxylation: Isocitrate is oxidized to alpha-ketoglutarate, producing NADH and releasing CO₂.
(Step 4) Further Decarboxylation: Alpha-ketoglutarate is further oxidized to succinyl-CoA, again producing NADH and CO₂. Hydrogen atoms released and picked up by coenzymes NAD⁺ and FAD. NAD⁺ picks up two electrons and one proton, reducing to NADH; FAD accepts two hydrogen atoms to be reduced to NADH₂
Now, all three carbon atoms in pyruvic acid are released as CO₂. Conversion of all six carbon atoms to CO₂ is completed in two turns of Krebs cycle.
Substrate-level Phosphorylation: Succinyl-CoA is converted to succinate, generating ATP (or GTP). Guanosine Triphosphate serves as an intermediary at this point.
Oxidation: Succinate is oxidized to fumarate, producing FADH₂.
Hydration: Fumarate is hydrated to malate.
Final Oxidation: Malate is oxidized to regenerate oxaloacetate, producing NADH. This oxaloacetate can then combine with another acetyl-CoA to repeat the cycle.
Products: The Krebs cycle produces 6 NADH, 2 FADH₂, 2 ATP (or GTP), and 4 CO₂ molecules. These electron carriers (NADH and FADH₂) are crucial for the electron transport chain, where most ATP is produced during aerobic respiration.
Describe the chemiosmotic mechanism of ATP generation.
Electron Transport Chain System: a sequence of carrier molecules that are capable of oxidation and reduction. As electrons pass through the chain, there is a release of energy to drive the chemiosmotic generation of ATP, making the final oxidation irreversible. Eukaryotes have it in the inner mitochondrial membrane and prokaryotes have it in the plasma membrane. Three classes of carrier models in electron transport chains:
Flavoproteins: contain flavin and are capable of performing oxidations and reductions, one important one being flavin mononucleotide (FMN). Can transfer both protons and electrons.
Ubiquinones (Coenzyme Q): small nonprotein carriers. Can transfer protons and electrons.
Cytochromes: Proteins with an iron-containing protein (-heme). The iron can be reduced (Fe²⁺) or oxidized (Fe³⁺). They can only transfer electrons Cytochromes in this chain include:
cytochrome b (cyt b)
cytochrome c₁ (cyt c₁)
cytochrome c (cyt c)
cytochrome a (cyt a)
cytochrome a₃ (cyt a₃)
In the electron transport chain system:
Electrons are transferred from NADH to FMN, the first carrier. Hydrogen atom with 2 electrons passes to FMN which picks up another hydrogen ion in the aqueous solution. NADH is oxidized to NAD⁺ and FMN is reduced to FMNH₂.
FMNH₂ passes 2H⁺ to other side of mitochondrial membrane and two electrons to Q. FMNH₂ is oxidized to FMN and Q picks up an addition 2H⁺ from surrounding membrane and releases it to other side of membrane.
Electrons are passed successively to cyt b, cyt c₁, cyt c, cyt a, and cyt a₃. Each cytochrome is reduced and oxidized. The last cytochrome passes the electrons to oxygen, which becomes negatively charged before picking up protons to form water. As you progress along the chain, each electron carrier has greater electronegativity before it. Since they can only transfer electrons, the buildup of protons provides enough energy for chemiosmotic mechanism for ATP generation.
Chemiosmosis mechanism: it involves oxidative phosphorylation. Energy released as a substance moves along a concentration gradient is used to generate ATP. The steps are:
As energetic electrons from NADPH pass down transport chain, some carriers (proton pumps) actively transport protons across membrane.Q transports electrons between first and second complexes, and cyt c transports them between second and third complexes.
Phospholipid membrane is impermeable to protons, so it establishes a gradient. The excess H⁺ on one side makes it more positively charged than another. The electrochemical gradient has potential energy, called proton motive force.
Protons on higher concentration side can only diffuse across membrane through protein channels that contain ATP synthase. Energy is released to synthesize ADP into ATP as protons flow.
Compare and contrast aerobic and anaerobic respiration.
Anerobic respiration: the final receptor is an inorganic molecule other than oxygen, rarely an organic molecule. Some bacteria, Pseudomonas and Bacillus, can use nitrate ion (NO3-) as final receptor to become nitrite ion (NO2-), nitrous oxide, or nitrogen gas. Other bacteria, Desulfovibrio, use sulfate (SO42-). Some archaea use CO2 to form methane. Sulfur and Nitrogen use are essential to nitrogen and sulfur cycles. Amount of ATP is varies, but never reaches as high as aerobic respiration.
Aerobic respiration: The final electron receptor is oxygen. The various transfers in the electron transport chain generate about 34 molecules of ATP from each glucose oxidized, three from each of ten molecules of NADH (total of 30) and two from each of the two FADH₂ (total of four). Aerobic respiration in prokaryotes generates 38 molecules of ATP: 34 from chemiosmosis plus four generated by oxidation in glycolysis and Krebs cycle. Eukaryotes produce two fewer ATPs because NADH cannot enter mitochondria.
Describe the chemical reactions of, and list some products of, fermentation.
Fermentation can convert pyruvic acid into an organic product, having NAD⁺ and NADP⁺ regenerated to enter another round of glycolysis. Fermentation
5.5
Describe how lipids and proteins undergo catabolism.
Provide two examples of the use of biochemical tests to identify bacteria in the laboratory.
Compare and contrast cyclic and noncyclic photophosphorylation.
Compare and contrast the light-dependent and lightindependent reactions of photosynthesis.
Compare and contrast oxidative phosphorylation and photophosphorylation.
Write a sentence to summarize energy production in cells.
Categorize the various nutritional patterns among organisms according to carbon source and mechanisms of carbohydrate catabolism and ATP generation.
Describe the major types of anabolism and their relationship to catabolism.
Define amphibolic pathways.