Chapter 10 - Cerebrospinal Fluid
Formation and Physiology
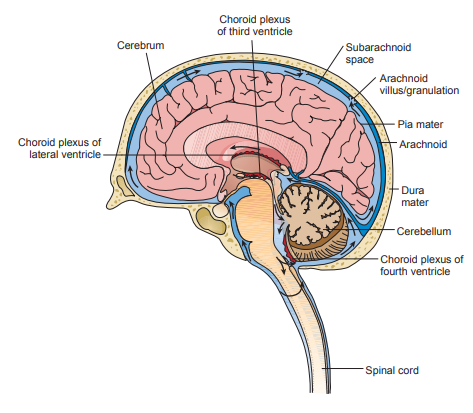
Cerebrospinal fluid (CSF) supplies nutrients to nervous tissue, removes metabolic waste, and produces a mechanical barrier to cushion the brain and spinal cord.
The fluid flows through the subarachnoid space located between the arachnoid and pia mater.
- The arachnoid is a filamentous (spiderlike) inner membrane.
- The pia mater is a thin membrane lining the surfaces of the brain and spinal cord.
CSF is produced in the choroid plexuses of the two lumbar ventricles and the third and fourth venticles.
- In adults, approximately 20 mL of fluid is produced every hour, but it gets reabsorbed at an equal rate in the arachnoid granulations/villae.
- A volume of 90 to 150 mL in adults and 10 to 60 mL in neonates needs to be maintained.
- The cells of the arachnoid granulations act as one-way valves that respond to pressure within the central nervous system (CNS) and prevent reflux of the fluid.
- The choriod plexuses are capillary networks that form the CSF from plasma by mechanisms of selective filtration under hydrostatic pressure and active transport secretion.
- In the choroid plexuses, the endothelial cells have very tight-fitting junctures that prevent the passage of many molecules, making a blood-brain barrier.
Specimen Collection and Handling
- CSF is routinely collected by lumbar puncture between the third, fourth, or fifth lumbar vertebrae.
- The volume of CSF that can be removed is based on the volume available in the patient (adult vs. neonate) and the opening pressure of the CSF taken when the needle first enters the subarachnoid space.
- Elevated pressure requires fluid to be withdrawn slowly to prevent too much volume.
- Specimens are collected in three sterile tubes, which are labeled 1, 2 and 3 in the order in which they are withdrawn.
- Tube 1 is used for chemical and serologic tests because these tests are least affected by blood or bacteria introduced as a result of the tap procedure; they are stored frozen.
- Tube 2 is usually designated for the microbiology laboratory and are stored at room temperature.
- Tube 3 is used for the cell count, because it is the least likely to contain cells introduced by the spinal tap procedure; they are stored in the refrigerator.
- A fourth tube may be drawn for the microbiology laboratory to provide better exclusion of skin contamination or for additional serologic tests.
- Supernatant fluid that is left over after each section has performed its tests may also be used for additional chemical or serologic tests.
- Excess fluid should not be discarded and should be frozen until there is no further use for it.
Appearance
- A cloudy, turbid, or milky specimen can be the result of an increased protein or lipid concentration, but it may also be indicative of infection, with the cloudiness being caused by the presence of WBCs.
- Pink, orange or yellow specimens are xanthochromic and are caused by the presence of RBC degradation products.
- It depends on the amount of blood and the length of time it has been present, from pink (very slight amount of oxyhemoglobin) to orange (heavy hemolysis) to yellow (conversion of oxyhemoglobin to unconjugated bilirubin).
- Specimens that contain up to 200 WBCs or 400 RBCs/μl may appear clear, so it is necessary to examine all specimens microscopically.
- Grossly bloody CSF can be an indication of intracranial hemorrhage, but it may also be due to the puncture of a blood vessel during the spinal tap procedure.
- Blood from a cerebral hemorrhage will be evenly distributed throughout the three tubes, whereas a traumatic tap gradually diminishes from tube 1 to 3.
- Bloody CSF caused by intracranial hemorrhage does not contain enough fibrinogen to clot while a traumatic tap can.
- Detection of the fibrin degradation product, D-dimer, by latex agglutination immunoassay indicates the formation of fibrin at a hemorrhage site.
- RBCs must usually remain in the CSF for approximately 2 hours before noticeable hemolysis begins so a traumatic tap is usually clear; however, a very recent hemorrhage would also produce a clear supernatant.
- To examine a bloody fluid for the presence of xanthochromia, the fluid should be centrifuged in a microhematocrit tube and the supernatant examined against a white background.
- The microscopic finding of macrophages containing ingested RBCs (erythrophagocytosis) or hemosiderin granules is indicative of intracranial hemorrhage.
- Other causes of xanthochromia include elevated serum bilirubin, presence of the pigment carotene, markedly increased protein concentrations and melanoma pigment.
- Xanthochromia that is caused by bilirubin due to immature liver function is also commonly seen in infants, particularly in those who are premature.
| Appearance | Cause | Major Significance |
|---|---|---|
| crystal clear | normal | |
| hazy, turbid, milky | microorganisms | meningitis |
| protein | disorders of the blood-brain barrier | |
| production of IgG within the CNS | ||
| cloudy | WBCs | meningitis |
| oily | radiographic contrast media | |
| bloody | RBCs | hemorrhage |
| traumatic tap | ||
| xanthochromic | hemoglobin | old hemorrhage |
| lysed cells from traumatic tap | ||
| bilirubin | RBC degradation | |
| increased serum bilirubin | ||
| carotene | increased serum carotene | |
| protein | disorders of the blood-brain barrier | |
| melanin | meningeal melanosarcoma | |
| clotted | protein | disorders of the blood-brain barrier |
| clotting factors | introduced by traumatic tap | |
| contains a pellicle | protein | disorders of the blood-brain barrier |
| clotting factors | tubercular meningitis |
Cell Count
The cell count that is routinely performed on CSF specimens is the leukocyte (WBC) count.
- Normal adult CSF contains 0 to 5 WBCs/μL, with a higher number in children, and as many as 30 mononuclear cells/μL can be considered normal in newborns.
Any cell count should be performed immediately, because WBCs (particularly granulocytes) and RBCs begin to lyse within 1 hour, with 40% of the leukocytes disintegrating after 2 hours.
- Specimens that cannot be analyzed immediately should be refrigerated.
Methodology
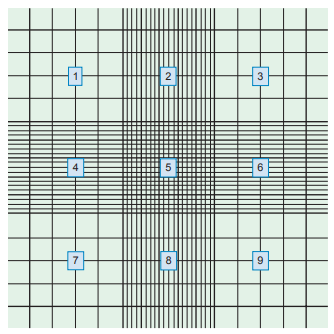
A Neubauer counting chamber is routinely used for performing CSF cell counts.
They must be soaked in a bactericidal solution for at least 15 minutes and then thoroughly rinsed with water and cleaned with isopropyl alcohol.
Clear specimens may be counted undiluted, provided no overlapping of cells is seen during the microscopic examination.
Dilutions for total cell counts are made with normal saline mixed by inversion.
If only WBCs are needed, 3% glacial acetic acid is used for dilution to lyse the RBCs, along with methylene blue to stain and differentiate neutrophils and mononuclear cells.
Cells are counted in the four corner squares and the center square on both sides of the hemocytometer.
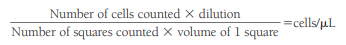
Corrections for Contamination
To correct for WBCs and protein artificially introduced into the CSF from a traumatic tap, the CSF RBC count and the blood RBC and WBC counts are needed.
The number of artificially added WBCs can be calculated using the following formula:
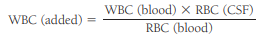
An approximate CSF WBC count can then be obtained by subtracting the “added” WBCs from the actual count.
Quality Control of Cerebrospinal Fluid and Other Body Fluid Cell Counts
- On a biweekly basis, all diluents should be checked for contamination by examination in a counting chamber under 4x magnification.
- Contaminated diluents should be discarded and new solutions prepared.
- On a monthly basis, the speed of the cytocentrifuge should be checked with a tachometer, and the timing should be checked with a stopwatch.
Differential Count on a Cerebrospinal Fluid Specimen
The differential count should be performed on a stained smear and be different from the sample used in the counting chamber.
To ensure that the maximum number of cells are available for examination, the specimen should be concentrated prior to the preparation of the smear.
Cytocentrifugation
- Fluid is added to the conical chamber, and as the specimen is centrifuged, cells present in the fluid are forced into a monolayer within a 6-mm diameter circle on the slide and absorbed by the filter paper blotter, producing a more concentrated area of cells.
- As little as 0.1 mL of CSF combined with one drop of 30% albumin produces an adequate cell yield when processed with the cytocentrifuge.
- Addition of albumin increases the cell yield and decreases the cellular distortion frequently seen on cytocentrifuged specimens.
- Positively charged coated slides to attract cells are also available.
- Cells from both the center and periphery of the slide should be examined because cellular characteristics may vary between areas of the slide.
- A daily control slide for bacteria should also be prepared using 0.2 mL saline and two drops of the 30% albumin currently being used.
- Cellular distortion may include cytoplasmic vacuoles, nuclear clefting, prominent nucleoli, indistinct nuclear and cytoplasmic borders and cellular clumping that resembles malignancy.
- The chamber count should be repeated if too many cells are seen on the slide, and a new slide should be prepared if not enough cells are seen on the slide.
Cerebrospinal Fluid Cellular Constituents
| Type of Cell | Microscopic Findings | Major Clinical Significance |
|---|---|---|
| lymphocytes | all stages of development may be found | normal |
| viral, tubercular and fungal meningitis | ||
| multiple sclerosis | ||
| neutrophils | disintegrate rapidly | bacterial meningitis |
| early viral, tubercular and fungal meningitis | ||
| cerebral hemorrhage | ||
| monocytes | mixed with lymphocytes | normal |
| viral, tubercular and fungal meningitis | ||
| multiple sclerosis | ||
| macrophages | may contain RBCs appearing as empty vacuoles or ghost cells, hemosiderin granules and hematoidin crystals | RBCs in spinal fluid |
| radiographic contrast media | ||
| blast forms | lymphoblasts, myeloblasts or monoblasts | acute leukemia |
| lymphoma cells | resemble lymphocytes with cleft nuclei | disseminated lymphomas |
| plasma cells | in traditional and classic forms | multiple sclerosis |
| lymphocyte reactions | ||
| ependymal, choroidal and spindle-shaped cells | in clusters with distinct nuclei and distinct cell walls | diagnostic procedures |
| malignant cells | in clusters with fusing of cell borders and nuclei | metastatic carcinomas |
| primary CNS carcinoma |
Adults usually have a predominance of lymphocytes to monocytes (70:30), whereas the ratio is reversed in children; occasionally, neutrophils are also present.
- Increased numbers of cells indicates pleocytosis.
- If it involves neutrophils, lymphocytes, or monocytes, it is most frequently associated with an infection of the meninges (meningitis).
Neutrophils
Following cytocentrifugation, neutrophils may also contain cytoplasmic vacuoles and have no granules.
Granules are also lost more rapidly in CSF.
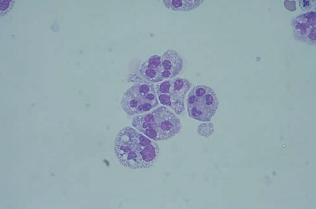
Neutrophils with pyknotic or shrunken nucleii indicate degenerating cells and resemble nucleated red blood cells with multiple nucleii.
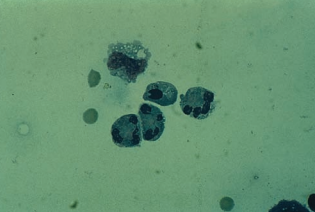
A high CSF WBC count with increased neutrophils is considered indicative of bacterial meningitis.
Increased neutrophils are also seen in the early stages (1 to 2 days) of viral, fungal, tubercular and parasitic meningitis.
Neutrophils associated with bacterial meningitis may contain phagocytized bacteria.
Neutrophils may also be increased following central nervous system (CNS) hemorrhage, repeated lumbar punctures, and injection of medications or radiographic dye; however, these are of little significance.
Lymphocytes and Monocytes
A moderately elevated CSF WBC count with a high percentage of lymphocytes and monocytes suggests meningitis of viral, tubercular, fungal, or parasitic origin.
Reactive lymphocytes containing increased dark blue cytoplasm and clumped chromatin are frequently present during viral infections.
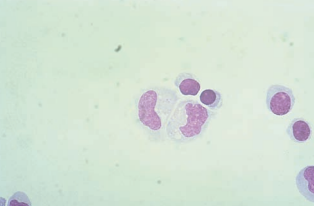
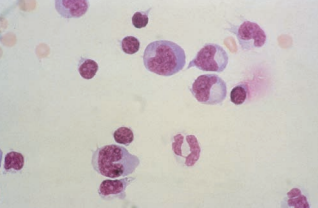
Increased lymphocytes are seen in cases of both asymptomatic HIV infection and AIDS.
A moderately elevated WBC count (less than 50 WBCs/μL) with increased normal and reactive lymphocytes and plasma cells may be indicative of multiple sclerosis or other degenerative neurologic disorders.
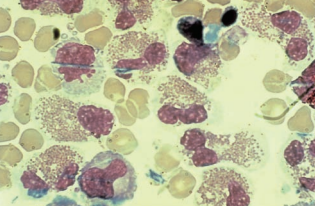
Eosinophils
Increased eosinophils are associated with parasitic infections, fungal infections (primarily Coccidioides immitis), and introduction of foreign material, including medications and shunts into the CNS.
Macrophages
Macrophages appear within 2 to 4 hours after RBCs enter the CSF and are frequently seen following repeated taps.
They tend to have more cytoplasm than monocytes in the peripheral blood.
The finding of increased macrophages is indicative of a previous hemorrhage.
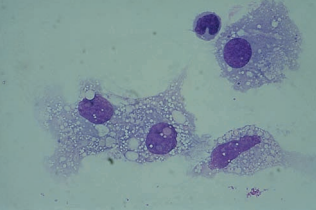
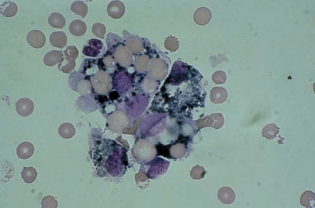
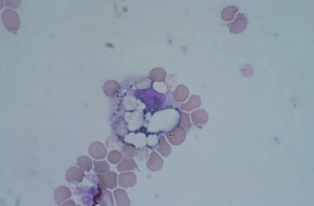
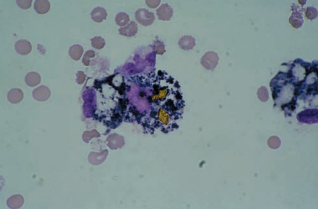
Further degradation of the phagocytized RBCs results in the appearance of dark blue or black iron-containing hemosiderin granules.
Yellow hematoidin crystals represent further degeneration.
They are iron-free, consisting of hemoglobin and unconjugated bilirubin.
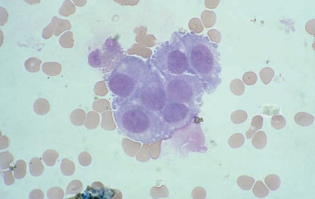
Nonpathologically Significant Cells
These cells are most frequently seen following diagnostic procedures such as pneumoencephalography and in fluid obtained from ventricular taps or during neurosurgery.
Choroidal cells are from the epithelial lining of the choroid plexus.
They are seen singularly and in clumps with distinct cell borders.
Nucleoli are usually absent and nuclei have a uniform appearance.
Ependymal cells are from the lining of the ventricles and neural canal.
They have less defined cell membranes and are frequently seen in clusters.
Nucleoli are often present.
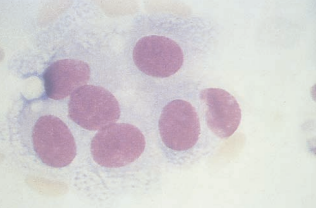
Spindle-shaped cells represent lining cells from the arachnoid.
They are ususally seen in clusters and may be seen with systemic malignancies.
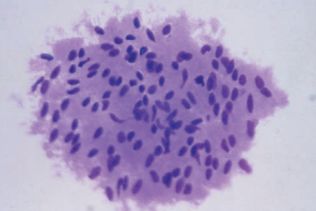
Malignant Cells of Hematologic Origin
Lymphoblasts, myeloblasts and monoblasts in the CSF are frequently seen as a serious complication of acute leukemias.
Nucleoli are often more prominent than in blood smears.
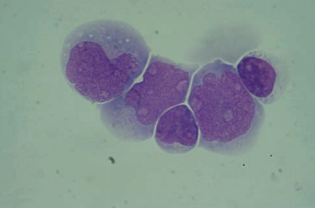
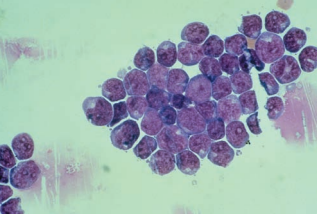
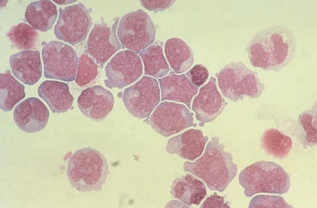
Lymphoma cells are also seen in the CSF indicating dissemination from the lymphoid tissue.
They resemble large and small lymphocytes and usually appear in clusters of large, small, or mixed cells based on the classification of the lymphoma.
Nuclei may appear cleaved, and prominent nucleoli are present.
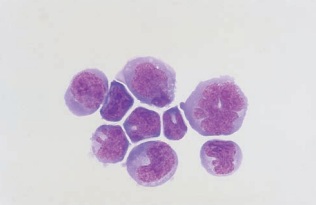
Malignant Cells of Nonhematologic Origin
Metastatic carcinoma cells of nonhematologic origin are primarily from lung, breast, renal, and gastrointestinal malignancies.
Cells from primary CNS tumors include astrocytomas, retinoblastomas, and medulloblastomas.
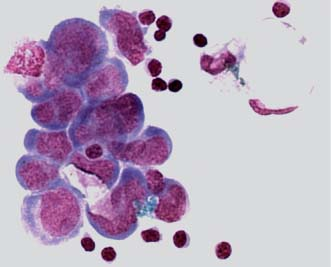
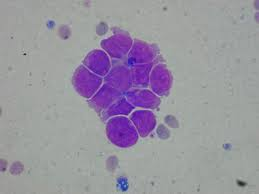
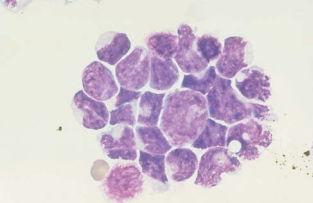
They usually appear in clusters and must be distinguished from normal clusters of ependymal, choroid plexus, lymphoma, and leukemia cells.
Fusing of cell walls and nuclear irregularities and hyperchromatic nucleoli are seen in clusters of malignant cells.
Chemistry Test
The clinically important CSF chemicals are few, but under certain conditions, it may be necessary to measure a larger variety.
Cerebrospinal Fluid Protein
Normal values for total CSF protein are usually listed as 15 to 45 mg/dL, but are somewhat method dependent, and higher values are found in infants and older persons.
Elevated levels are caused by damage to the blood-brain barrier, production of immunoglobulins within the CNS, decreased clearance of normal protein from the fluid, degeneration of neural tissue, meningitis and hemorrhage conditions.
- Protein that appears in the CSF as a result of damage to the integrity of the blood-brain barrier contains fractions proportional to those in plasma.
- Diseases that stimulate the immunocompetent cells in the CNS show a higher proportion of IgG.
Decreased levels are caused by fluid leaking from the CNS.
Plasma protein can be artificially introduced into a specimen by a traumatic tap, so corrections to calculation must be made.
Both the cell count and the protein determination must be done on the same tube.
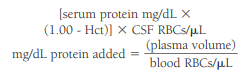
CSF proteins are measured using principles of turbidity production or dyebinding ability.
- The turbidity method has been adapted to automated instrumentation in the form of nephelometry.
- Methods for the measurement of CSF protein are available for most automated chemistry analyzers.
Albumin makes up the majority of CSF protein, while prealbumin is the second most prevalent fraction in CSF.
The major alpha globulins include haptoglobin and ceruloplasmin, the major beta globulin is transferrin; and the major gamma globulin is immunoglobulin G (IgG).
Only a small amount of IgA is present.
A separate carbohydratedeficient transferrin fraction, referred to as “tau,” is only present in CSF and used to confirm suspicious specimens collected.
Immunoglobulin M (IgM), fibrinogen, and beta lipoprotein are not present.
To accurately determine whether IgG is increased because it is being produced within the CNS or is elevated as the result of a defect in the blood-brain barrier, comparisons between serum and CSF levels of albumin and IgG must be made.
The CSF/serum albumin index evaluates the integrity of the blood-brain barrier.
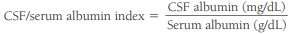
An index value less than 9 represents an intact blood-brain barrier, and it increases relative to the amount of damage to the barrier
The CSF IgG index measures IgG synthesis within the CNS.
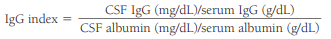
In general, values greater than 0.70 are indicative of IgG production within the CNS.
Electrophoresis
- The primary purpose for performing CSF protein electrophoresis is for the detection of oligoclonal bands representing inflammation within the CNS.
- The bands are located in the gamma region of the protein electrophoresis, indicating immunoglobulin production.
- To ensure that the oligoclonal bands are present as the result of neurologic inflammation, simultaneous serum electrophoresis must be performed.
- Leukemia, lymphoma, and viral infections produce serum and CSF banding, indicating blood-brain barrier leakage or traumatic introduction of blood into the CSF specimen.
- The presence of two or more oligoclonal bands in the CSF that are not present in the serum indicates multiple sclerosis, particularly when accompanied by an increased IgG index, and other neurologic disorders such as encephalitis, neurosyphilis, Guillain-Barré syndrome and neoplastic disorders.
- CSF must be concentrated if protein levels are low before performing electrophoresis.
- Agarose gel electrophoresis is followed by Coomassie brilliant blue staining.
- Isoelectric focusing (IEF) is followed by silver staining.
- Immunofixation electrophoresis (IFE) is more sensitive and does not require concentration.
Myelin Basic Protein
- The presence of myelin basic protein (MBP) in the CSF is indicative of recent destruction of the myelin sheath that protects the axons of the neurons (demyelination).
- Measurement of the amount of MBP in the CSF can be used to monitor the course of multiple sclerosis.
- It may also provide a valuable measure of the effectiveness of current and future treatments. Immunoassay techniques are used for measurement.
Cerebrospinal Fluid Glucose
- Glucose enters the CSF by selective transport across the blood-brain barrier, which results in a normal value that is approximately 60% to 70% that of the plasma glucose.
- Elevated CSF glucose values are always a result of plasma elevations.
- The finding of a markedly decreased CSF glucose accompanied by an increased WBC count and a large percentage of neutrophils is indicative of bacterial meningitis.
- If the WBCs are lymphocytes instead of neutrophils, tubercular meningitis is suspected.
- If a normal CSF glucose value is found with an increased number of lymphocytes, the diagnosis would favor viral meningitis.
- Other causes of decreased CSF glucose include alterations in the mechanisms of glucose transport across the blood-brain barrier and by increased use of glucose by the brain cells.
- The blood glucose should be drawn about 2 hours prior to the spinal tap to allow time for equilibration between the blood and fluid.
- CSF glucose is analyzed using the same procedures employed for blood glucose.
- Specimens should be tested immediately because glycolysis occurs rapidly in the CSF.
Cerebrospinal Fluid Lactate
- In bacterial, tubercular, and fungal meningitis, the elevation of CSF lactate to levels greater than 25 mg/dL occurs much more consistently than does the depression of glucose and provides more reliable information when the initial diagnosis is difficult.
- Levels greater than 35 mg/dL are frequently seen with bacterial meningitis.
- In viral meningitis, lactate levels remain lower than 25 mg/dL.
- CSF lactate levels remain elevated during initial treatment but fall rapidly when treatment is successful, thus offering a sensitive method for evaluating the effectiveness of antibiotic therapy.
- Other causes of increased CSF lactate include destruction of tissue within the CNS due to oxygen deprivation (hypoxia) and severe head injuries.
- RBCs contain high concentrations of lactate, and falsely elevated results may be obtained on xanthochromic or hemolyzed fluid.
Cerebrospinal Fluid Glutamate
- Glutamine is produced from ammonia and α-ketoglutarate by the brain cells to prevent toxicity from building-up in the CNS.
- The normal concentration of glutamine in the CSF is 8 to 18 mg/dL.
- Elevated levels are found in association with liver disorders that result in increased blood and CSF ammonia.
- As the concentration of ammonia in the CSF increases, the supply of α-ketoglutarate becomes depleted; glutamine can no longer be produced to remove the toxic ammonia, and coma ensues usually at levels more than 35 mg/dL.
- Approximately 75% of children with Reye syndrome have elevated CSF glutamine levels.
- Several methods of assaying glutamine are available and are based on the measurement of ammonia liberated from the glutamine.
- This is preferred over the direct measurement of CSF ammonia because the concentration of glutamine remains more stable than the concentration of volatile ammonia in the collected specimen.
- The CSF glutamine level also correlates with clinical symptoms much better than does the blood ammonia.
Microbiology Tests
The CSF culture is actually a confirmatory rather than a diagnostic procedure; however, several methods are available to provide information for a preliminary diagnosis.
Gram Stain
Specimens are concentrated by centrifugation at 1500 g for 5 minutes, because often only a few organisms are present at the onset of the disease.
However, at least a 10% chance exists that Gram stains and cultures will be negative; thus, blood cultures should be taken, because the causative organism is often present in both the CSF and the blood.
Bacteria commonly found such as Streptococcus pneumoniae, Haemophilus influenzae, Escherichia coli and Neisseria meningitidis can be detected using enzyme-linked immunosorbent assay (ELISA) or bacterial antigen test (BAT).
Streptococcus agalactiae and Listeria monocytogenes may also be encountered in newborns.
Acid-fast or fluorescent antibody stains are not routinely performed on specimens unless tubercular meningitis is suspected.
In cases of fungal meningitis, an India ink preparation is performed to detect the presence of thickly encapsulated Cryptococcus neoformans with a classic starburst pattern.
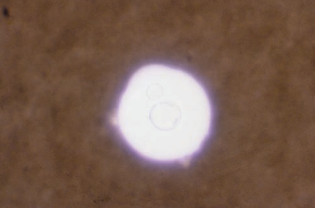
Latex agglutination tests may also be used but false-positive interferences may occur from interactions with rheumatoid factors.
Commercial kits that make use of incubation with dithiothreitol or pronase and boiling with ethylenediaminetetra-acetic acid can also be used with less false-positive interference.
Serologic Testing
- Serologic testing is performed to detect the presence of neurosyphilis.
- The procedure recommended by the CDC to diagnose neurosyphilis is the Venereal Disease Research Laboratories (VDRL); however, it is not as sensitive as the fluorescent treponemal antibody-absorption (FTA-ABS) test for syphilis.
- If the FTA-ABS is used, care must be taken to prevent contamination with blood, because it remains positive in the serum of treated cases of syphilis.
- The rapid plasma reagin (RPR) test is not recommended for use on CSF, because it is less sensitive and specific than the VDRL.
- To prevent unnecessary testing, a positive serum test should be obtained using the FTA-ABS.
- Fluid can be frozen until serum results are available.
Meningitis Diagnosis
| bacterial | viral | tubercular | fungal |
|---|---|---|---|
| w/ neutrophils | w/ lymphocytes | w/ lymphocytes and monocytes | w/ lymphocytes and monocytes |
| marked protein elevation | moderate protein elevation | moderate to marked protein elevation | moderate to marked protein elevation |
| marked glucose decrease | normal glucose level | glucose decrease | normal to decreased glucose |
| lactate >35 mg/dL | normal lactate level | lactate >25 mg/dL | lactate >35 mg/dL |
| positive Gram stain and bacterial antigen tests | w/ pellicle | positive India ink and immunologic test for C. neoformans |