Redox
Key Vocabulary:
Oxidation:
Gain of oxygen or loss of electrons by a species.Reduction:
Loss of oxygen or gain of electrons by a species.Oxidising Agent:
Causes another substance to be oxidised by accepting electrons (itself is reduced).Reducing Agent:
Causes another substance to be reduced by donating electrons (itself is oxidised).Spectator Ions:
Ions that do not change during the reaction – same charge and state throughout.

Redox Reactions
Redox reactions are reactions that involve oxidation and reduction.
Oxidation and reduction were originally defined in terms of oxygen:
Oxidation is the gain of oxygen
Reduction is the loss of oxygen
Example:
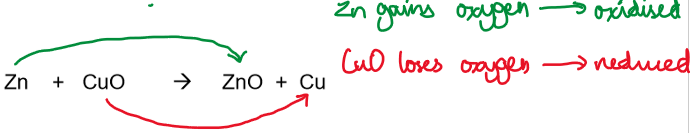
When it was realised that ionic compounds could be written as ions, it became clear that the oxide ion in the above reaction was a spectator ion and could be left out.
The equation then becomes:
Zn + Cu²⁺ → Zn²⁺ + Cu
Oxidation and reduction in this equation cannot be defined in terms of oxygen, but are defined by transfer of electrons:
Zn is oxidised (loses electrons)
Cu²⁺ is reduced (gains electrons)
Remember the formula:
Otherwise known as oxidation is loss (OIL), reduction is gain (RIG)

Ionic Equations and Redox (Summary)
An ionic equation includes only the species directly involved in the reaction (ions, covalent compounds, or elements).
Aqueous ionic compounds and acids can be broken into ions (e.g., CaSO₄ → Ca²⁺ + SO₄²⁻).
Spectator ions appear unchanged on both sides and are cancelled out.
The final ionic equation shows only reacting species.
To identify oxidation or reduction, check for changes in oxidation state (charge).
Constructing an ionic equation:
Balanced symbol equation:
Fe(s) + 2AgNO₃(aq) → Fe(NO₃)₂(aq) + 2Ag(s)Convert ionic compounds into ions:
Fe(s) + 2Ag⁺(aq) + 2NO₃⁻(aq) → Fe²⁺(aq) + 2NO₃⁻(aq) + 2Ag(s)Cancel spectator ions:
Remove 2NO₃⁻(aq) (same on both sides)Final ionic equation:
Fe(s) + 2Ag⁺(aq) → Fe²⁺(aq) + 2Ag(s)
Fe is oxidised (loses electrons)
Ag⁺ is reduced (gains electrons)
Determining which species are reduced/oxidised:
The ionic equation shows which species is oxidised or reduced.
Focus on the left-hand side (reactants):
The Ag⁺ is being reduced because it changes to Ag, which is more negative – it gains electrons.
The Fe is being oxidised because it changes to Fe²⁺, which is more positive – it loses electrons.
Tasks:
1. copper(II) oxide + hydrogen → copper + water
Balanced symbol equation: CuO + H₂ → Cu + H₂O
Species oxidised: H₂
How can you tell? Becomes H⁺ – loses electrons
Species reduced: Cu²⁺
How can you tell? Becomes Cu – gains electrons
2. zinc oxide + carbon → zinc + carbon dioxide
Balanced symbol equation: ZnO + C → Zn + CO₂
Species oxidised: C
How can you tell? Forms CO₂ – gains oxygen
Species reduced: Zn²⁺
How can you tell? Forms Zn – loses oxygen (gains electrons)
3. magnesium + hydrochloric acid → magnesium chloride + hydrogen
Balanced symbol equation: Mg + 2HCl → MgCl₂ + H₂
Balanced ionic equation: Mg + 2H⁺ → Mg²⁺ + H₂
Species oxidised: Mg
How can you tell? Becomes Mg²⁺ – loses electrons
Species reduced: H⁺
How can you tell? Becomes H₂ – gains electrons
4. iron + copper(II) sulfate → iron(II) sulfate + copper
Balanced symbol equation: Fe + CuSO₄ → FeSO₄ + Cu
Balanced ionic equation: Fe + Cu²⁺ → Fe²⁺ + Cu
Species oxidised: Fe
How can you tell? Becomes Fe²⁺ – loses electrons
Species reduced: Cu²⁺
How can you tell? Becomes Cu – gains electrons
5. potassium bromide + fluorine → bromine + potassium fluoride
Balanced symbol equation: 2KBr + F₂ → Br₂ + 2KF
Balanced ionic equation: 2Br⁻ + F₂ → Br₂ + 2F⁻
Species oxidised: Br⁻
How can you tell? Becomes Br₂ – loses electrons
Species reduced: F₂
How can you tell? Becomes F⁻ – gains electrons
Example question:
In Example 5:
Reaction:
2KBr + F₂ → Br₂ + 2KF
Ionic equation:
2Br⁻ + F₂ → Br₂ + 2F⁻
Which species is the oxidising agent?
F₂ – it gains electrons (is reduced), so it causes Br⁻ to be oxidised.
Which species is the reducing agent?
Br⁻ – it loses electrons (is oxidised), so it causes F₂ to be reduced.
Writing Half Equations:
A half equation shows either oxidation or reduction and always includes electrons.
If a species is reduced, electrons are added to the reactant side.
If a species is oxidised, electrons are added to the product side.
Example:
Reaction:
Sodium iodide + bromine → sodium bromide + iodine
Step 1: Write a balanced chemical equation:
2NaI + Br₂ → 2NaBr + I₂
Step 2: Write a balanced ionic equation by removing spectator ions:
2I⁻ + Br₂ → 2Br⁻ + I₂
Step 3: separate the ionic equation in 2 half equations:
Oxidation: The species being oxidised (I⁻) loses electrons.
Reduction: The species being reduced (Br₂) gains electrons.
Step 4: add the appropriate number of electrons:
Oxidation (Iodine is oxidised):
2I⁻ → I₂ + 2e⁻
Reduction (Bromine is reduced):
Br₂ + 2e⁻ → 2Br⁻
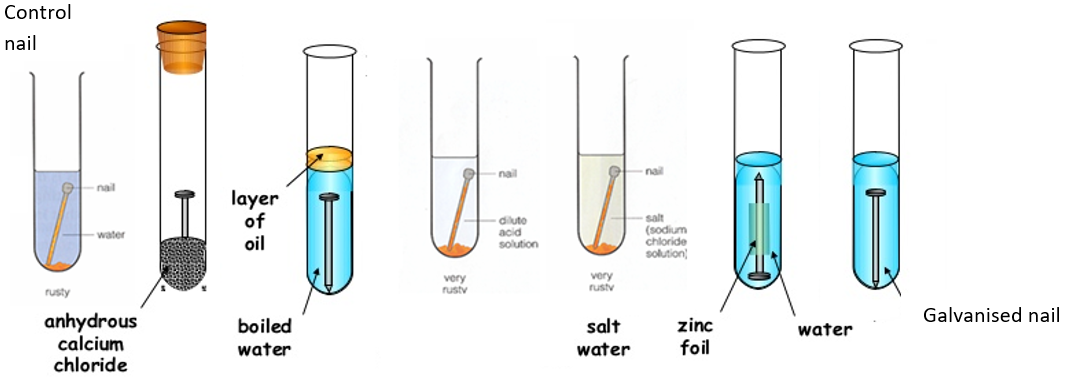
Rusting:
· The following test tubes were set up as described in the table.
· These are the results after leaving the tubes for one week.
· Use the diagram to help you complete the table below.
Results:
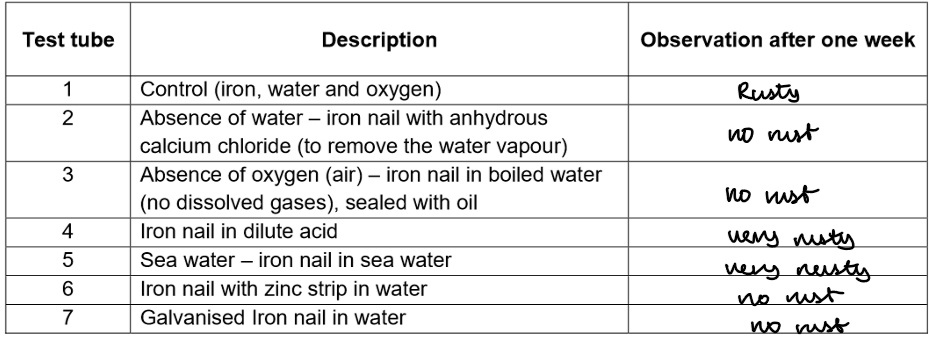
Conclusion
Rusting requires both oxygen and water.
Rusting is accelerated by the presence of salt and acid.
Rusting is prevented by the presence of protective coatings such as paint, oil, galvanization (zinc coating), or using alloys.
Word Equation:
Iron + Oxygen + Water → Hydrated iron(III) oxide (rust)
Fe₂O₃·xH₂O
Problems of rusting:
Corrosive
A substance that gradually destroys or damages materials through chemical reactions, often oxidation.
Can damage metals, concrete, and even human tissue.
Rusting
A type of corrosion affecting iron and its alloys, forming iron oxide (rust).
Occurs when iron reacts with oxygen in the presence of water or moisture.
Weakens the metal structure and makes it unsightly.
Iron and steel rust but are still widely used due to being cheap, strong, and versatile. Examples: bridges, piers, buildings, cars, machinery.
Rust flakes away, causing continuous rusting until the structure is destroyed.
Rusting reduces the strength of structures and makes them unsightly.
Millions of pounds are spent annually on replacing parts and preventing rust.
Preventing Rusting
Physical Barrier
Prevents water/oxygen from reaching the metal.
Advantage: Cheap, easy to apply.
Disadvantage: If scratched or removed, rusting occurs.
Examples:
(i) Painting – ships, vehicles, bridges
(ii) Oiling and greasing – machines, bicycle chains
(iii) Plastic coating – draining racks in kitchens
Sacrificial Protection
More reactive metals (e.g., magnesium or zinc) are used to corrode instead of iron/steel.
Advantage: Useful for hard-to-reach places (e.g., underground pipes).
Disadvantage: Sacrificial blocks need to be replaced regularly.
Examples:
Magnesium blocks on oil rigs (North Sea)
Zinc blocks on iron ship hulls
Bags of scrap magnesium on underground pipes
Galvanising
Iron/steel coated with zinc, providing both a physical barrier and sacrificial protection.
Advantage: Long-lasting protection.
Disadvantage: Zinc layer must remain intact.
Examples:
Zn(s) → Zn²⁺(aq) + 2e⁻
Applications: Nails, motorway crash barriers, car body panels, bins, buckets
Alloying
Alloys mix additional metals to improve properties and prevent rust.
Example: Stainless steel (70% Fe, 20% Ni, 10% Cr) – used in cutlery where the additional metals prevent rust but increase cost.