Chapter 1: Structure and Bonding (McMurry Organic Chemistry, 9th Edition)
What Is Organic Chemistry?
All living systems are composed of organic chemicals.
• Proteins (e.g., hair keratin)
• Genetic material (DNA)
• Foods, drugs, polymers, etc.Defined as the study of carbon compounds; >50\times10^{6} known molecules contain C.
Unique features of carbon
• ext{Group 4A} element → 4 valence e^- → 4 covalent bonds
• C–C bonding yields long chains/rings ⇒ unparalleled structural diversity.
Historical Origins
Mid-1700s: chemists isolated low-melting, difficult-to-purify substances from plants & animals and called them “organic.”
Vitalism: the theory that organic compounds can only be produced by living organisms or through a “vital force” and cannot by synthesized from inorganic materials—laboratory synthesis deemed impossible.
Scientists believed it was not possible to create organic compounds (compounds containing carbon, like those found in living things) in a laboratory from non-living, inorganic materials.
1816 (Chevreul): Chevreul found that soap can be separated into several organic compounds, which he termed “fatty acids.”
These are the reasons why he called them “fatty acids.”
They came from fat: He discovered these compounds by breaking done soap, which is made from animal or vegetable fats (these fats are made of molecules called triglycerides).
The compounds that he separated from the soap had acidic characteristics (they could react with bases)
They were oily or greasy in nature (these acids had long hydrocarbon chains, making them non-polar and giving them a fat-like consistency and appearance; this is the reason they are called fatty acids)
1828 (Wöhler): Wöhler showed that it was possible to convert inorganic salt ammonium cyanate into organic substance urea
Periodic Context of Carbon
Carbon is located in Period 2, Group 4A (carbon is the 14th column from the left in the periodic table - it is in group 14)
Neighboring non-metallic elements (B, N, O, F) strongly influence bonding trends in organic chemistry.
Atomic Structure
The Nucleus
The core of an atom is dense and positively charged
It is composed of both protons and neutrons
Protons and neutrons account for nearly all of the atom’s mass
• Protons: +1 charge
• Neutrons: 0 chargeSurrounded by orbiting electrons that are ~~10^{-10}\text{ m} away from the nucleus of the atom (or 0.1 nanometers away)
Typical atomic diameter ≈ 2\times10^{-10}\text{ m}=200\text{ pm (picometers)} (1 Å = 10^{-10}\text{ m}).
Å (the unit angström)
Atomic Number & Mass
Z : Atomic number = number of protons (defines the element).
A : Mass number = protons + neutrons (mass number).
Isotopes: Atoms with the same atomic number but different mass numbers.
Atoms of a given element have the same atomic number.
Atomic mass/weight: weighted average (amu) of all isotopes.
Orbitals & Wave Functions
Schrödinger wave equation—A mathematical equation which describes the behavior of a specific electron in an atom.
The solutions (the answer) to the Schrödinger wave equation are called wave functions
For the hydrogen atom, these wave functions are also known as orbitals.
Orbitals is denoted (\psi).
|\psi|^{2} plot - describes where an eectron is most likely to be
An electron cloud has no specefic boundary - it is a “cloud” that has fuzzy boundaries.
The fuzziness means there’s no sharp edge - the electron doesn’t have a fixed path or exact position
There’s a probability of finding the elctron in a certian area - this areaforms the elctron cloud
Due to qunatum mechanics, we cannot know the exact postion and momentum of an electron at the same - Heisenberg Uncertainty Principle
Types & Shapes of Orbitals
s: spherical (nucleus at center).
p: dumbbell shaped with the nucleus at the middle
d: cloverleaf shaped, with four lobes, nuclues at center
Shells & Capacity
Orbtials in atoms are organized into different electron shells
Electrons occupy shells of rising energy (1, 2, 3…).
It is centered around the nucleus
Different shells contain different numbers and kinds of orbitals
Each orbital can be occupied by two electrons - Pauli Exclusion Principle
Capacities: 1st = 2 e^-, 2nd = 8, 3rd = 18 …
Each orbital holds max 2 e^- (opposite spins).
Each orbital can hold up to two electrons, but those electrons must have opposite spins; two electrons in the same orbital cannot have the same spin
Electron Configuration Rules
The overall purpose of electron configuration is to tell how many electrons are “spinning around” (orbiting) an atom of an element, and how they are arranged in different energy levels and orbitals - in NEUTRAL ATOMS (atoms that have no charge)
In non - neutral atoms (ions) you can determine how many electrons it has by look at its charge
Examples:
Na (neutral sodium atom)
Atomic number = 11 - 11 protons
Neutral - 11 electrons
Na+ (sodium ion)
Charge = +1 - lost 1 electron
Electrons = 11 - 1 = 10 electrons
Aufbau principle: fill lowest-energy orbitals first — order: 1s\rightarrow2s\rightarrow2p\rightarrow3s\rightarrow3p\rightarrow4s\rightarrow3d.
Pauli exclusion: no two e^- in the same orbital have identical quantum numbers; requires paired but opposite spins.
Hund’s rule: for degenerate orbitals, one e^- occupies each with parallel spin before pairing.
Degenerate orbitals are orbitals that have the same energy level
Worked Example: Sulfur
Atomic number = 16, electrons = 16
Sulfur is a neutral atom because no charge is shown
Configuration: 1s^{2}\,2s^{2}\,2p^{6}\,3s^{2}\,3p^{4}.
Worked Example: Magnesium Valence
Mg in Group 2A ⇒ 2 outer-shell electrons.
Development of Chemical-Bonding Theory
1850s (Kekulé, Couper): Independently observed that carbon is tetravalent.
It always forms four bonds when it joins other elements to form stable compounds
Carbon can bond to one another to form extended chains of linked atoms
Carbon can double back on themselves to form rings of atoms
1874 (Van’t Hoff, Le Bel): Propsed that the four bonds of carbon are not oriented randomly but have specific spatial directions.
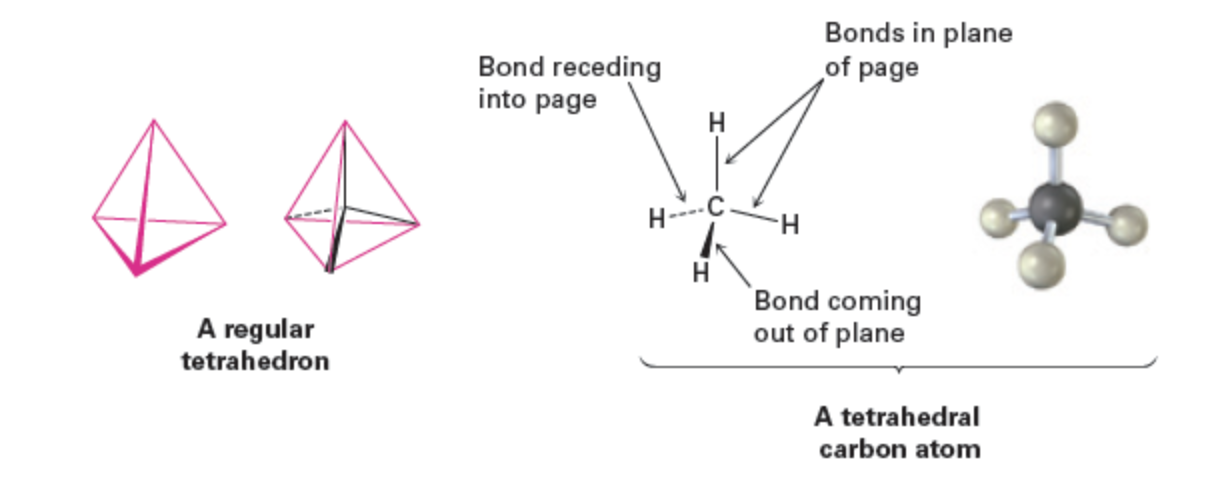
Van ‘t Hoff: Suggested that the four atoms to which carbon is bonded sit at the corners of a regular tetrahedron, with carbon in the center.
Why Bonds Form
Atoms form bonds because the resulting compounds is more stable than the separate atoms.
Basically, atoms form bonds because doing so makes them more stable than they were alone.
When aatoms share, gain or lose electrons to form chemical bonds, they reach this stable configuration.
The resulting compound has lower energy and is more stable than the separate atoms
Examples:
Two hydrogen atoms (H) each have 1 electron - they share to from H2 (alows them to be stable)
Sodium (Na) gives up 1 electrons to chlorine (Cl) - they from NaCl (allows them to be stable)
Remember this:
If an atom loses one electron (-1 charge gone), it now has one more proton than electron - net charge = +1
If an atom gains an extra electron (-1 charge added), it now has one more electron than proton
Valence shell - Atoms outermost shell
Bond types: These bonds form in the elcetron cloud of an atom
• Ionic bond: Ions held together by a electrostatic attraction; formed as a result of electron transfers
• Covalent bond: Formed by sharing electrons; organic compounds have covalent bonds from sharing electronsMolecule - Neutral collectron of atoms held toegther by covelent bonds
A non - neutral collection of atoms is held together by electrostatic forces or polar covalent bonds
Representations of Chemical Bonding
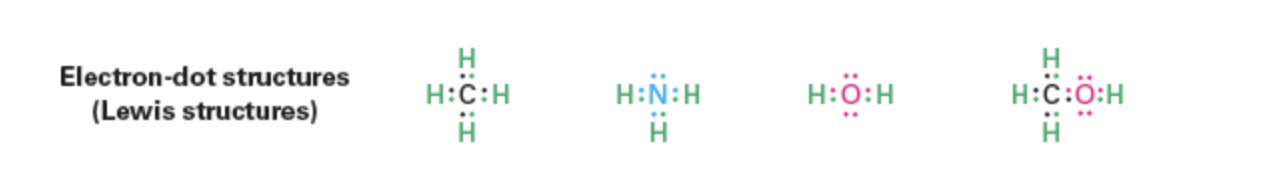
Lewis (electron-dot): Represents valence electrons as dots.
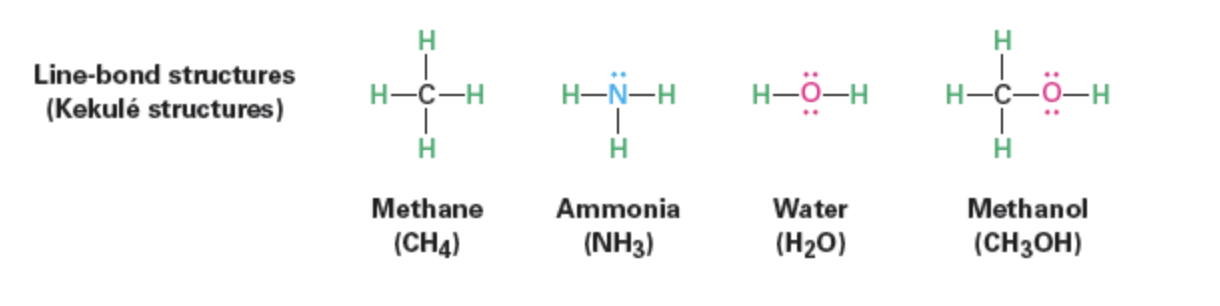
Kekulé (line-bond): Indicates two - electron covalent bond as a line drawn between atoms
Example set: methane, ammonia, water, methanol.
Octet & Typical Valences
The number of covalent bonds an atom forms depends on the number of additional valence electrons it needs to reach a stabel octet.
Carbon has four valence elctrons (2s2 2p2), forming 4 bonds, and needs 4 more to reach the neon configuration.
Nitrogen has five valence electrons (2s2 2p3), forming 3 bonds and needs 3 more electrons to achieve a full octet, resulting in a stable electronic configuration similar to neon.
Lone pair: valence electrons not involved in bonding
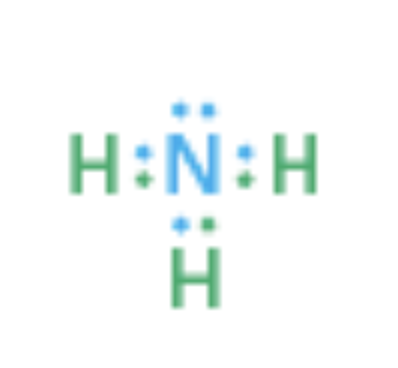
Example: Nitrogen atom in ammonia (NH_3 )
Shares six valence electrons in three covalent bonds
Two valence electrons are nonbonding lone pair
3-D Drawing Convention
Solid wedge: bond projecting toward viewer.
Dashed wedge: bond receding.
Plain line: bond in plane.
Worked problem: tetrahedral CHCl_3 drawing.
Valence-Bond (VB) Theory Fundamentals
Covalent bonds form when two atoms approach each other closely so that a singly occupied orbtial on one atom overlaps a singly occupied orbital in the other atom
H–H bond results from the overlap of two singly occupied hydrogen 1s orbitals - (1s–1s overlap) - For example: the molecule H2
H—H bond is cylindrically symmetrical forms a sigma (σ) bond
Bond energy released: 436\,\text{kJ mol}^{-1} → same as bond strength.
Bond strength - how strongly two atoms are held together
Bond length = the distacne between the nuclei of two bonded atoms; how far apart the centers of the atoms are
If the nuclei of both atoms are too close, they repel
If the nucleiu of both atoms are too far apart, bonding is weak
Hybridization & Molecular Geometry
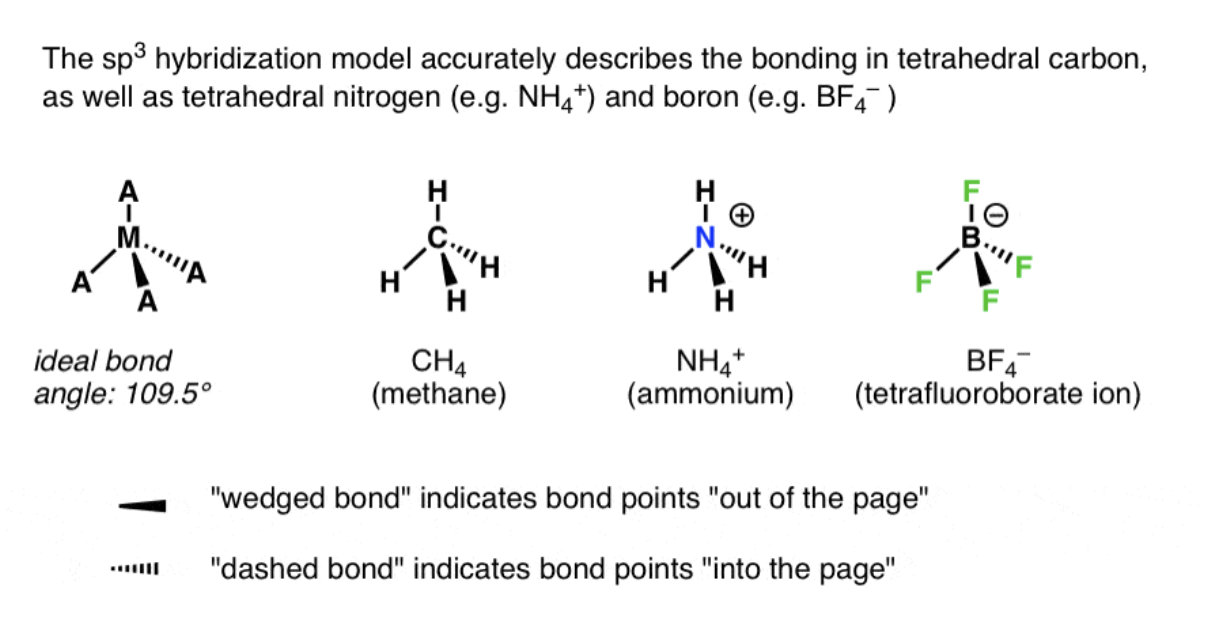
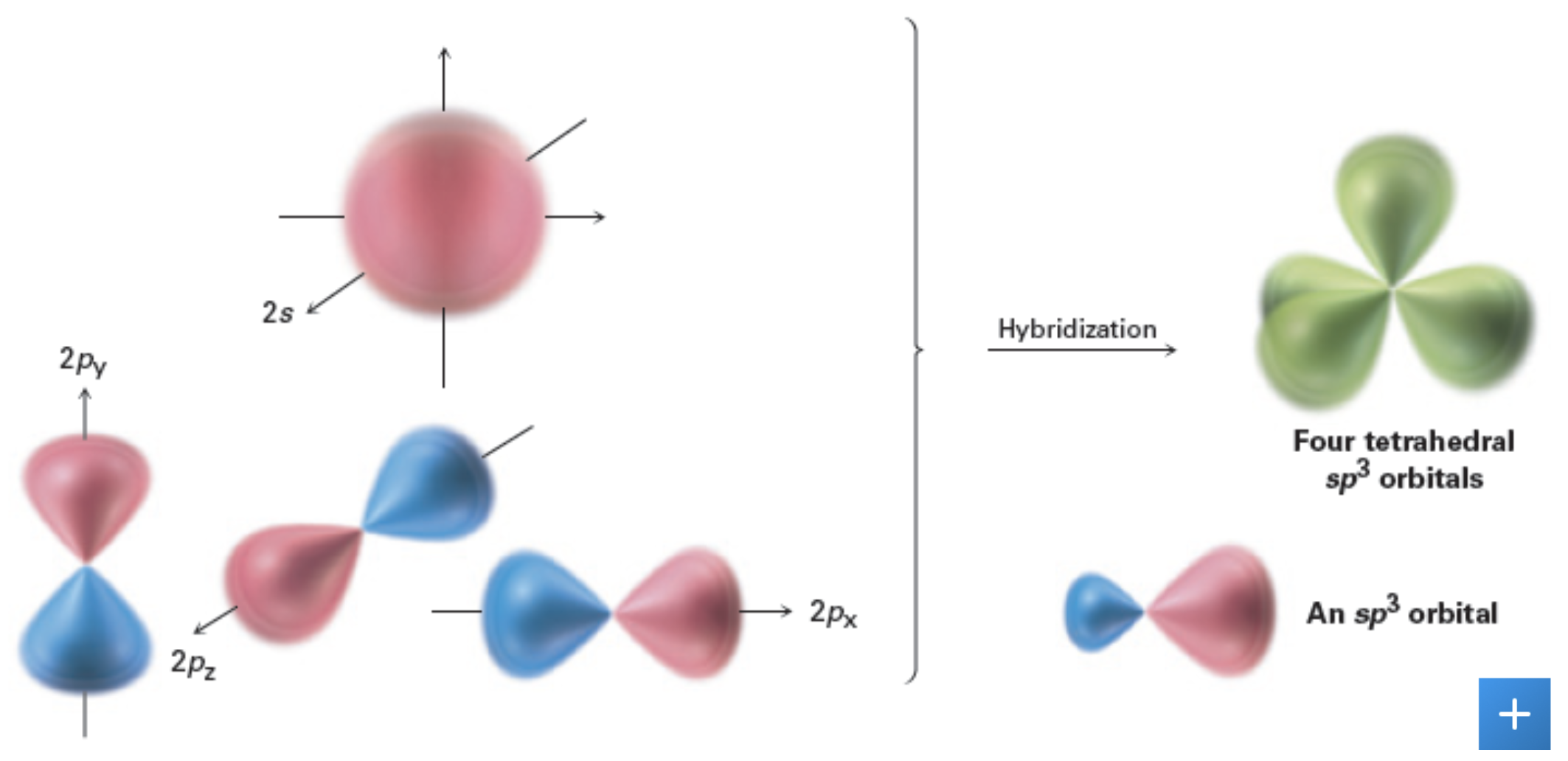
sp^3: Orbitals and the Structure of Methane
sp3 hybrid orbitals - hybrid orbitals derived by combination of an s atomic orbital with 3 p atomic orbitals; the 4 sp3 hybrids that result are directed toward the corners of a regular tetrahedron at angles of 109 degrees to each other.
Each C—H bond has a strength of 439 kJ/mol and a lenght of 109om
Bond angle - Formed between two adjacent bonds
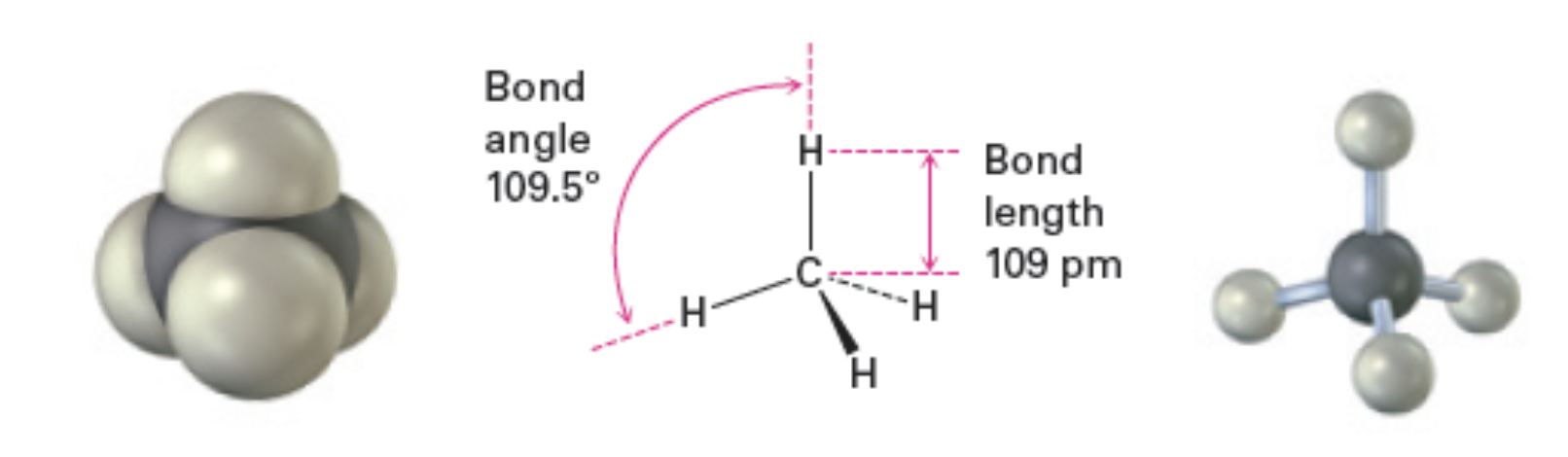
Bond angle = 109.5 degrees
Bond length = 109pm (picometers)
sp^2 : Ethylene
Hybridization: 1 s + 2 p → three coplanar sp^2 orbitals (120^{\circ} apart), plus unhybridized p_z.
sp: Acetylene
Heteroatom Hybridization (N, O, P, S)
Methylamine (CH3NH2): N sp^3; H–N–H 107.1^{\circ}, C–N–H 110.3^{\circ} (lone-pair compression).
Molecular-Orbital (MO) Theory Basics
Atomic orbitals combine → molecular orbitals delocalized over molecule.
For H_2:
• σ bonding MO (lower E, constructive).
• σ* antibonding MO (higher E, destructive; has a node).Electrons occupy lowest available MOs → only bonding MO filled in H_2.
π MOs: bonding from like-signed lobes, antibonding from opposite signs.
Drawing Organic Structures
Condensed & Skeletal Notation
In condensed formulas, C–H & C–C single bonds implicit (e.g., CH3CH2OH).
Skeletal (line-angle) rules:
• Carbons are vertices/line ends; hydrogens on carbon omitted.
• Heteroatoms & their hydrogens shown explicitly.Table 1.3 contrasts Kekulé vs skeletal for isoprene, methylcyclohexane, phenol.
Counting Hydrogens Example
Exercise on estrone skeleton: specify H count at each C & produce formula C{18}H{22}O_{2}.
Key Quantitative & Conversion Facts
Bond energy: 1\,\text{kJ} = 0.2390\,\text{kcal}; 1\,\text{kcal} = 4.184\,\text{kJ}.
Typical bond lengths:
• C–H (sp^3): \approx109\,\text{pm}.
• C–C (sp^3–sp^3): 154\,\text{pm}.
• C=C (sp^2–sp^2): shorter.
• C≡C (sp–sp): shortest.
Summary Highlights
Organic chemistry focuses on carbon’s tetravalency and hybridization versatility.
Atomic structure described by quantum orbitals; electron configurations obey Aufbau, Pauli, Hund.
Covalent bonding can be rationalized by VB (orbital overlap) or MO (orbital combination) theories.
σ bonds are head-on; π bonds are sideways overlaps of p orbitals.
Hybridizations dictate geometry:
• sp^3 (tetrahedral), sp^2 (trigonal planar), sp (linear).Heteroatoms (N, O, P, S) adopt hybridizations that maximize bond strength & lone-pair orientation.
Various structural shorthand (Lewis, Kekulé, condensed, skeletal) streamline the depiction of complex molecules while preserving critical bonding information.