Radioactivity + Particles
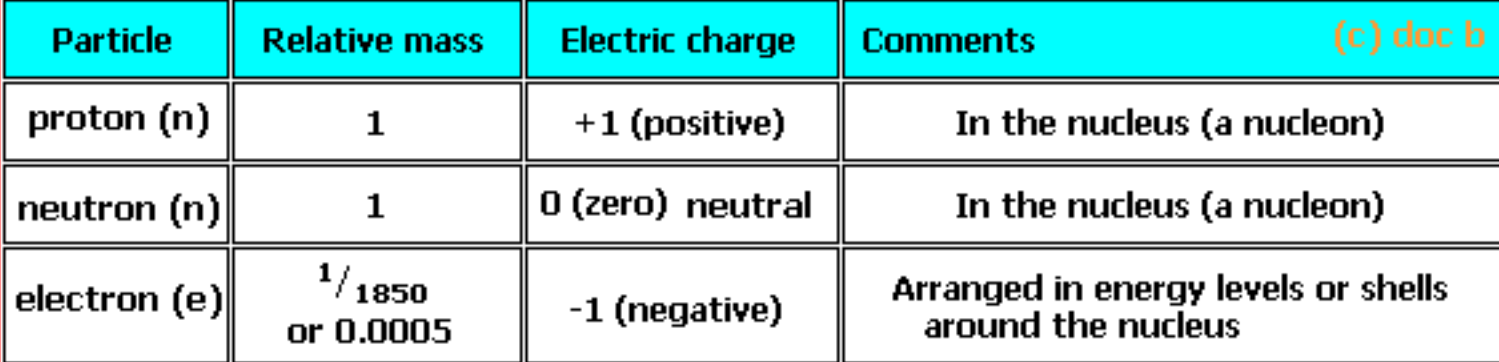 ISOTOPES are atoms with the same number of protons and electrons but different number of neutrons.
ISOTOPES are atoms with the same number of protons and electrons but different number of neutrons.
This means isotopes of an element have a different mass number, but the same atomic number.
Most elements have a few stable isotopes, but many other isotopes are unstable. 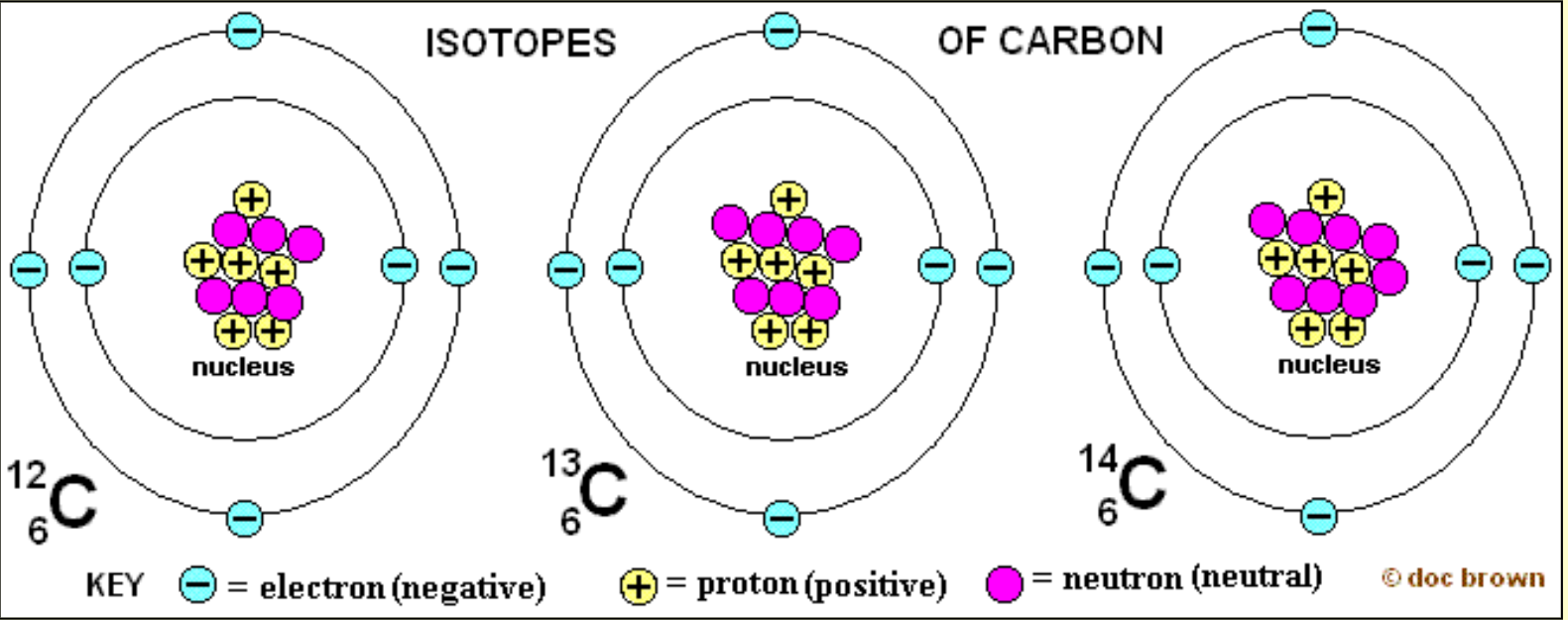 Radioactivity
Radioactivity
Radioactivity occurs when the unstable nucleus of an atom undergoes a fundamental change (disintegration). This results in a different nucleus being formed and accompanied by the emission of alpha particles or beta particles or gamma radiation.
Radioactive decay: the spontaneous disintegration of a nucleus which results in the nucleus emitting ionising radiation: alpha, beta, or gamma radiation.
Ionising radiations: knock electrons off atoms/molecules to create positive ions.
[[==Alpha particles==[[
[[A high velocity Helium nucleus of 2 protons and 2 electrons. It is expelled at high speeds (though still travels the slowest relatively) from the nucleus.[[
[[Mass = 4, charge = +2[[
- [[High ionising: biggest mass and highest charge = the biggest ‘punch’ in knocking off electrons from molecules to form other ions. It will attract and abstract 2 electrons from atoms, forming new ions.[[
- [[Low penetrating: biggest mass and highest charge, slowest speed. Stopped by a few cm of air or a thin sheet of paper.[[
<
<
<<^^Mass = 1/1850 (very small), charge = -1^^<<
- <<^^Moderate ionising: smaller mass and charge than alpha, but still quite good at knocking off electrons from molecules.^^<<
- <<^^Moderate penetrating: higher speed and middle values of mass and charge. Most stopped by a few mm of aluminium and will travel a few metres in air.^^<<
}}Gamma radiation}}
}}Very high-frequency electromagnetic radiation. Often accompanies alpha and beta decay. When a nucleus emits an alpha or beta particle, the daughter nucleus is usually left in an excited state. It can then move to a lower energy state by emitting a gamma ray.}}
}}The emission of a gamma photon in itself does NOT change the composition of the nucleus, it only lowers the energy associated with the nucleus.}}
}}Mass = 0, charge = 0}}
- }}Low ionising: carries no electric charge and has virtually no mass. So doesn’t create much of a ‘punch’ when colliding with an atom to knock off an electron.}}
- }}High penetrating: highest speed, smallest mass and charge. Most stopped by a thick layer of steel or concrete or dense lead. Can pass through many metres of air.}}
Contamination
Radioactive contamination: the unwanted presence of radioactive isotopes in the environment.
- Radioactive contaminants may be: in the air and breathed in, swallowed, or on surface you touch and absorbed through the skin.
- Contamination can be more dangerous than external irradiation, because the radioactive substance is actually inside your body and the nuclear radiation passes directly into the surrounding cell tissues.
Uses of Radiation
Kidney scans
Role: Used to diagnose certain kidney diseases - shows what kidneys look like and how well they work.
How: A tracer with a radioactive ‘tag’ (so it can be followed into the body with special detectors) is injected into the patient’s veins. A gamma camera is used to track the radioactivity.
Thyroid Cancer Radiotherapy
Role: Uses the radioisotope iodine 131 to kill thyroid cancer cells.
How: It circulates the patient’s body in their bloodstream, where thyroid cancer cells can pick up the iodine. The radiation in the iodine then kills the cancer cells.
Irradiation
Irradiation: when an object is exposed to ionising nuclear radiation from a radioactive source.
This doesn’t mean the irradiated object becomes radioactive.
- All radioactive emissions are extremely dangerous to living organisms. All three radiations can penetrate living cells, causing damage.
- If powerful enough, ionising radiation can cause burns, kill cells directly or cause genetic damage e.g. to the DNA molecules causing mutations and cancer.
If the radioactive source, a 'radionuclide', gets inside the body the 'danger' order is alpha > beta > gamma.
However, if the radioactive source is outside the body, the order danger is reversed to gamma > beta > alpha because the danger order follows the pattern of penetrating power.
Precautions to take
- Shielding: lead-lined aprons, stand behind a protective (lead/glass) screen, suit, face mask, gloves, goggles, apron
- Increasing you distance from the source (inverse square law)
- Encasing the source in certain materials to absorb harmful radiation
- Minimising spent time in the presence of the radioactive source
Most radiation occurs naturally, known as ‘background radiation’. Sources include:
- Radioactive minerals in the ground
- Cosmic rays from the sun
- Radon gas from rock, soil, and building minerals
- Food and water
- Building materials, rocks, and soil
Radiation sources due to Human Activity
- Emissions from nuclear power stations: the nuclear industry is legally allowed to emit tiny amounts of radioactive material into the environment.
- Radioisotope tracers used in industry and hospitals
- Nuclear accidents - like Chernobyl
- Atomic weapons testing
Detecting Radioactivity
Geiger-Muller tube
Instrument which electronically amplifies the effect of ionising radiation and is used for accurate measurements of radioactivity.
Can detect even a single radioactive event.
Records in counts per second.
The background radiation is measured and subtracted from any experimental laboratory results using radioisotopes - this is a 'fair test factor’ in investigations of radioactive materials.
Half lives
Some atomic nuclei are very unstable and only exist for a few microseconds, seconds, minutes, hours or days before decaying (disintegrating), these are known as radioisotopes.
- The breakdown of these unstable nuclei is called radioactive decay.
- Decay is a random event - you can't predict which isotope nucleus will disintegrate.
- You cannot alter the rate of the decay process - the radioactive atoms are totally unaffected by the physical or chemical state of the atoms - e.g. unaffected by change in temperature or pressure.
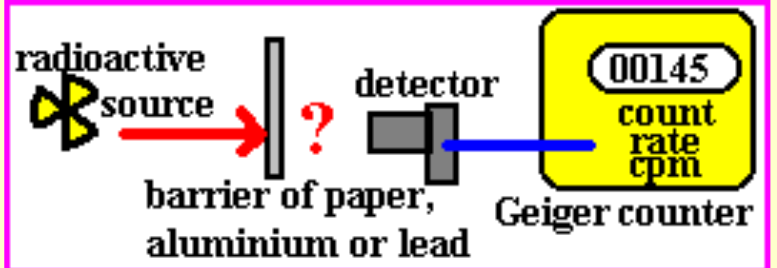
- There is a statistical pattern that the rate of decay follows - known as a ‘radioactive decay curve’.
- The graph of activity follows curve with a gradually decreasing negative gradient.
- If the gradient is steep, the more unstable is the isotope.
A measure of the stability of a radioactive isotope is given by its half-life.
^^Half life: the average time it takes for half of the remaining undecayed radioactive nuclei (atoms) to decay to a different nucleus (atom).^^
Radioactivity, or simply 'activity' is measured in becquerels (Bq).
- 1 becquerel = 1 disintegration or decay/second.
Radioisotopes used as tracers must have short half-lives, particularly those used in medicine to avoid the patient being dangerously over exposed to the harmful radiation, but a long enough half-life to enable accurate measurement and monitoring of the tracer.
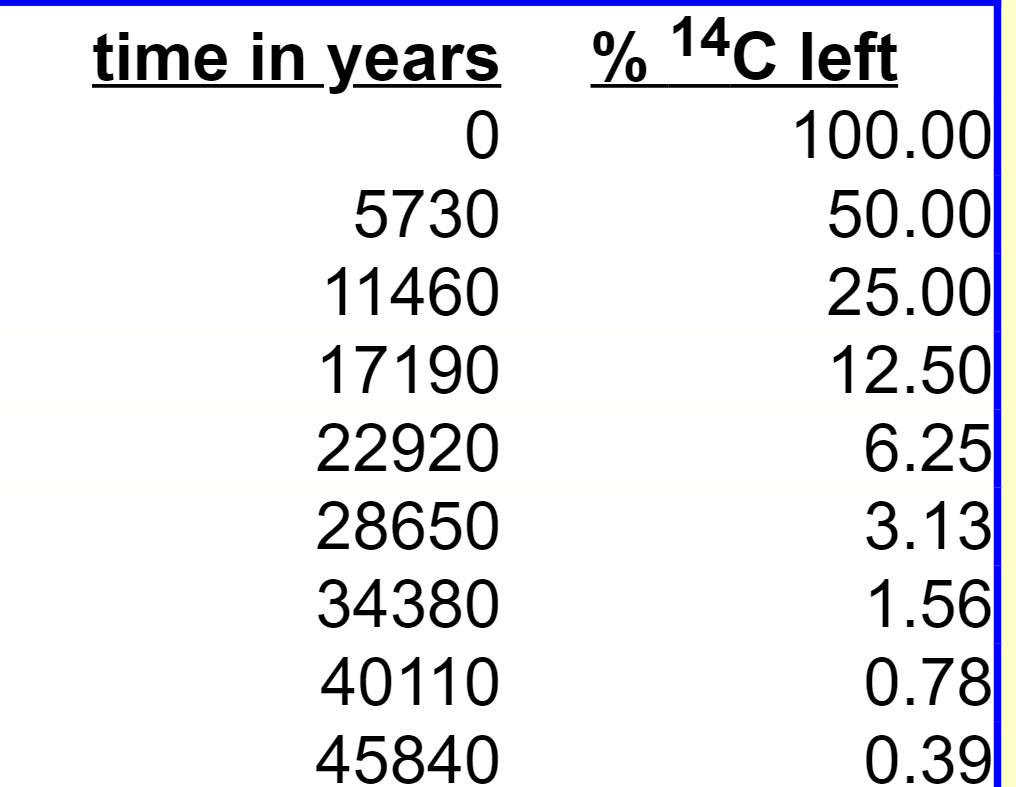
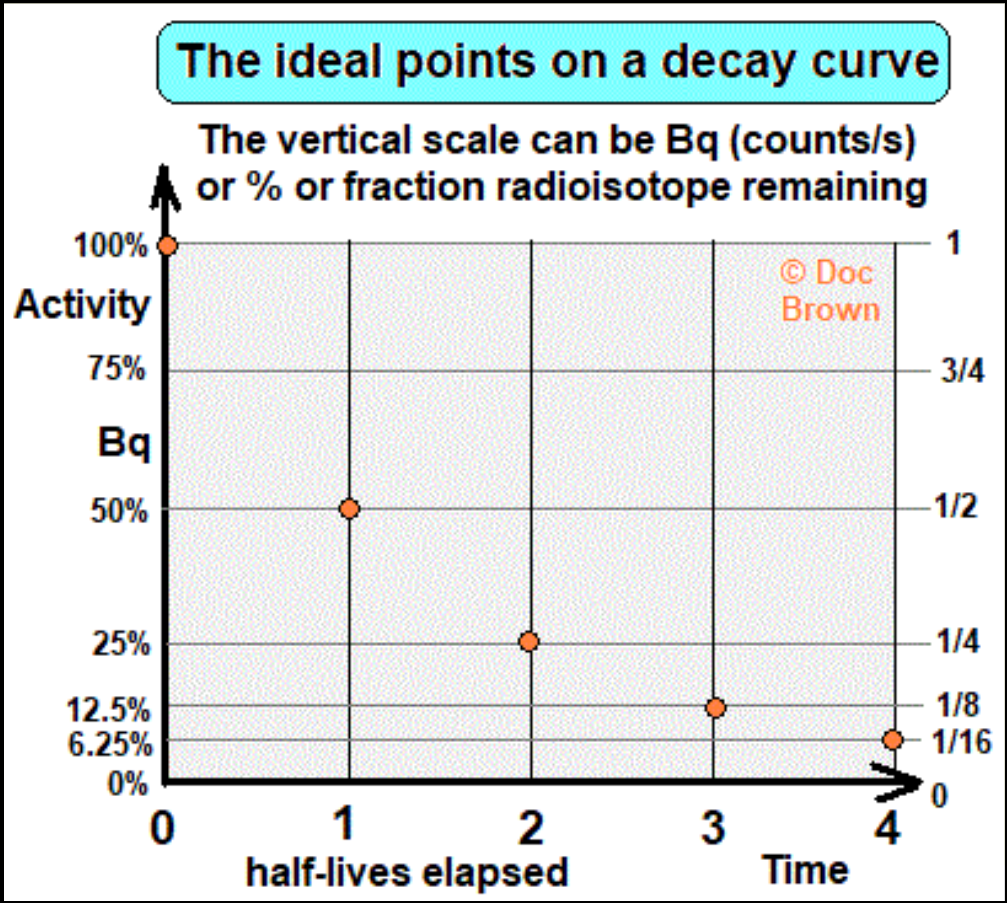 Carbon dating
Carbon dating
Carbon-14 is formed at a constant rate in the upper atmosphere by high energy nuclear processes. The carbon-14 atoms, like any other carbon atoms, become part of carbon dioxide in the atmosphere, and carbonates organic compounds in aquatic or land-based organisms.
Most carbon atoms are of the stable isotope carbon-12. A very small % of them are radioactive due to carbon-14 with a half-life of 5700 years. Archaeologists can use any material containing carbon of 'organic living' origin to determine its age.
- If an object has 1/2 (1/2 of 1, 50%) of the expected carbon-14 it must be 5700 years old,
- If it only has 1/4 (1/2 of a 1/2, 25%) of the expected 14C left, the object it must be 11400 years old (5 700 + 5 700),
- If only 1/8 (1/2 of 1/4, 12.5%) of the 14C left it is 17100 years old (11 400 + 5700)
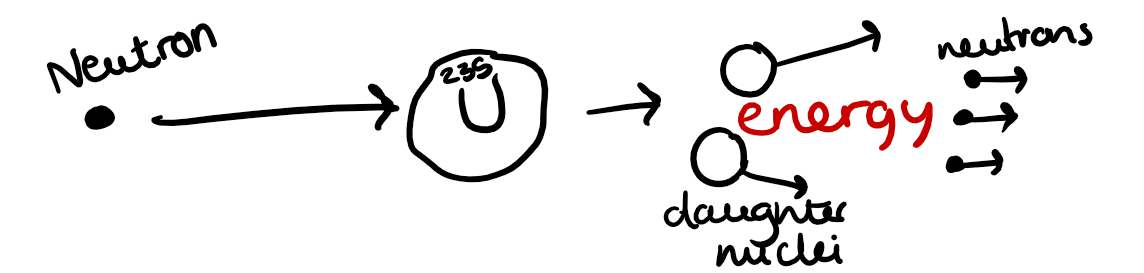
Nuclear Fission
When large atomic nuclei are hit with slow moving neutrons they can become highly unstable if the neutron is absorbed by the nucleus.
The larger unstable nucleus breaks into two smaller 'daughter' nuclei and also release more neutrons, as well as the production of beta and alpha particles and gamma radiation.
The neutrons must be slow moving to be absorbed by a uranium atom.
The heavier resulting nucleus is unstable and spontaneously breaks apart - nuclear fission, with the formation of several smaller atoms, neutrons and lots of nuclear energy released, which mainly ends up as heat energy. The mass loss is converted into energy e.g. thermal energy and electromagnetic radiation
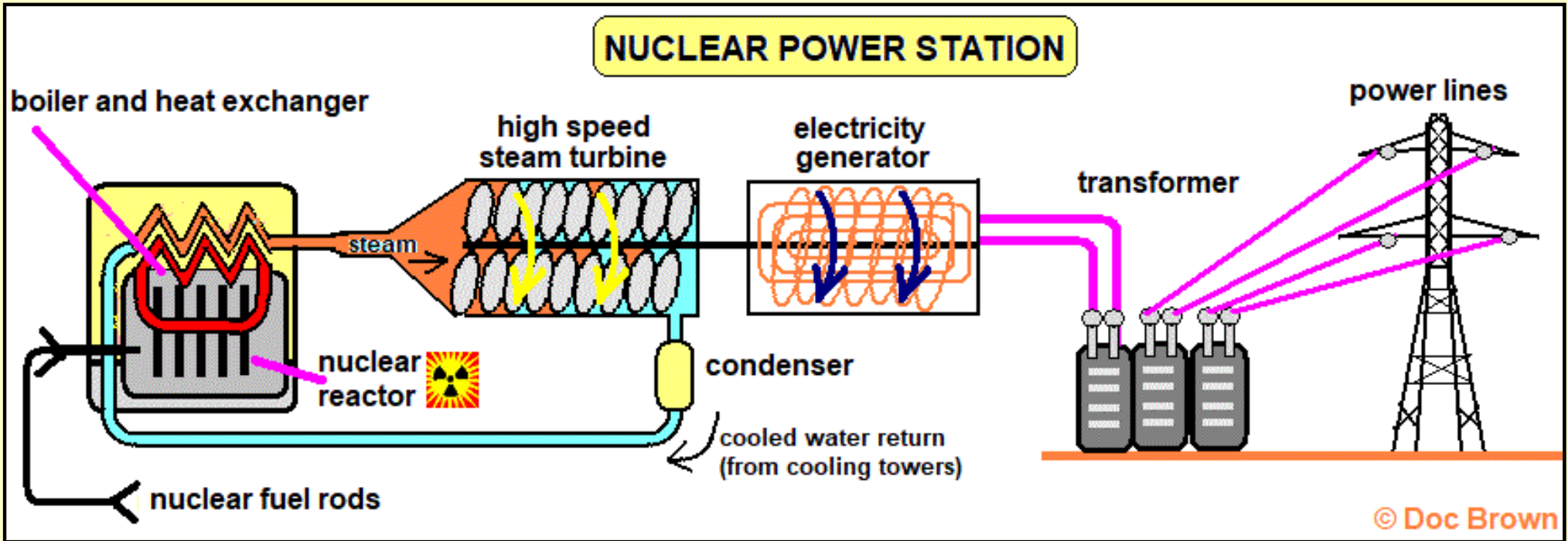
 The production of more neutrons that you started with (2-3 per uranium atom fission) go on to split more of the uranium atoms causing a chain reaction, which must be controlled to operate a nuclear reactor safely!
The production of more neutrons that you started with (2-3 per uranium atom fission) go on to split more of the uranium atoms causing a chain reaction, which must be controlled to operate a nuclear reactor safely!
Controls
Moderator - uses ^^water^^ (or graphite) to slow neutrons to a speed where they can be absorbed by the uranium nuclei. Speeds up the rate of fission.
Control rods - absorb neutrons to stop/slow the reaction. Often made of boron. Keeps the chain reaction and energy release under control. The boron moderator can be raised out of the reactor to allow more fission to take place.
Containment/shielding - keeps radiation in, or protects the reactor from damage.
Arguments for Nuclear Power
- It contributes little to the greenhouse effect and global warming since there is no emission of CO2 from fossil fuels. So climate change isn’t an issue.
- Provides a reliable large scale source of electricity
- provides lots of jobs in construction.
Arguments against Nuclear Power
- Radioactive leakages pose great danger to the environment and ourselves.
- Unsafe storage and disposal of harmful radioactive waste
- 10-20 year construction time
- Huge capital cost to build and maintain
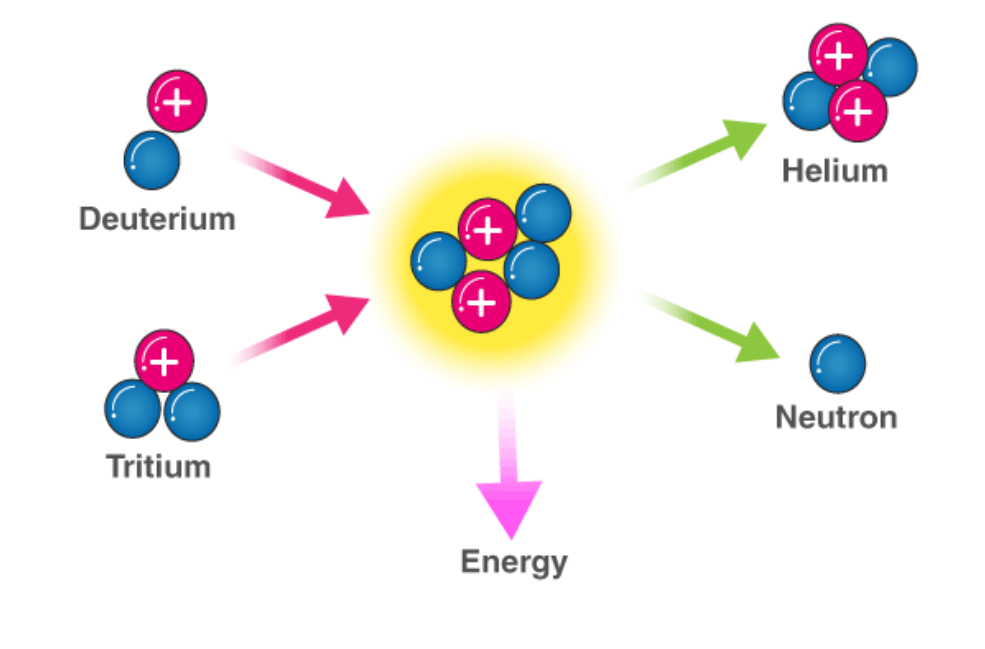
Nuclear Fusion
At the extremely high temperatures (10 million degrees) in the core of stars the atomic nuclei have such high KE that on collision they can fuse together - the nuclear process of fusion.
Requirements for fusion: Extremely high temperatures (and pressures) are needed to give the particles sufficiently high KE to overcome the giant repulsion forces of the two positive nuclei involved e.g. two positive hydrogen nuclei.
Process: Two smaller atomic nuclei fuse into one larger nucleus. This also releases massive amounts of energy.
- Either way a heavier nucleus is created.
Pros of Fusion
- Clean - doesn’t produce CO2
- Plentiful fuel - seawater
- If we manage to create fusion on earth, then it could become a cheap fuel option.
Cons of Fusion
- Need to have and maintain extremely high temperatures, making it very expensive and not yet viable.