Module 3: The structure of crystalline solids
Fundamental Concepts
Atoms in crystalline solids are positioned in orderly and repeated patterns that are in contrast to the random and disordered atomic distribution found in noncrystalline or amorphous materials
Unit cells
Crystal structures are specified in terms of parallelepiped units cells, which are characterized by geometry and atom positions within
Metallic crystal structures
Most common metals exist in at least one of three relatively simple crystal structures:
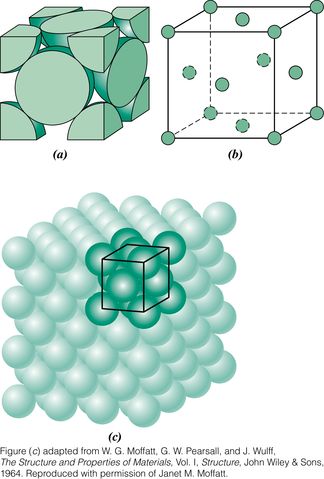
Face-centered cubic (FCC), which has a cubic unit cell
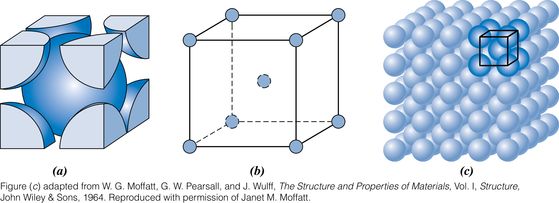
Body-centered cubic (BCC), which also has cubic unit cell
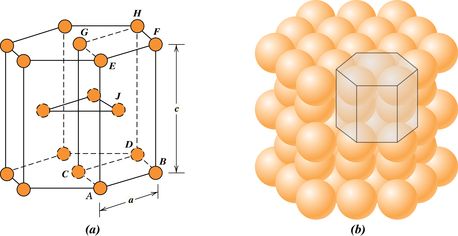
Hexagonal close-packed, which has a unit cell of hexagonal symmetry
Two features of a crystal structure are
Coordination number - the number of nearest-neighbor atoms
Atomic packing factor - the fraction of solid sphere volume in the unit cell
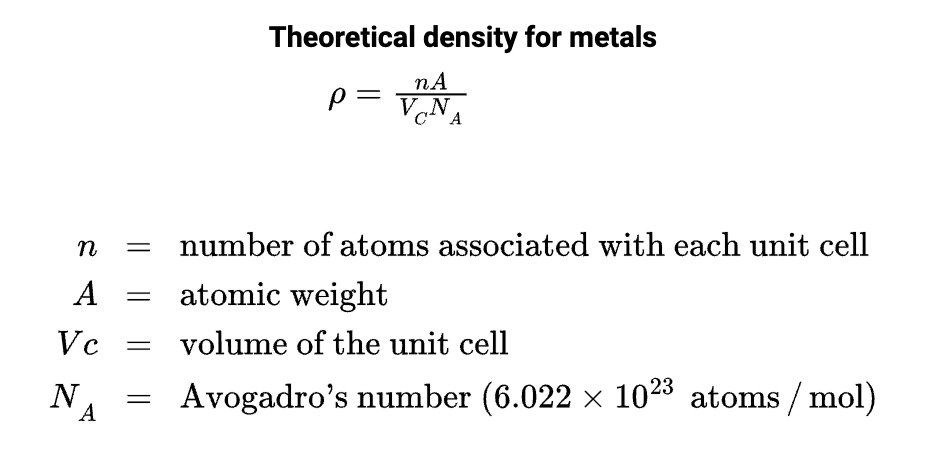
Density computations
The theoretical density of a metal is a function of the number of equivalent atoms per unit cell, the atomic weight, unit cell volume, and Avogadro’s number
Polymorphism and allotropy
polymorphism is when a specific material can have more than one crystal structure. Allotropy is polymorphism for elemental solids
Crystal systems
used to classify crystal structures on the basis of unit cell geometry
unit cell edge lengths and interaxial angles
there are seven crystal systems: cubic, tetragonal, hexagonal, orthorhombic, rhombohedral (trigonal), monoclinic, and triclinic
Point coordinates
crystallographic points, directions, and planes are specified in terms of indexing schemes
The basis for the determination of each index is a coordinate axis system defined by the unit cell for the particular crystal structure
Crystallographic directions
the location of a point within a unit cell is specified using coordinated that are fractional multiples of the cell edge lengths
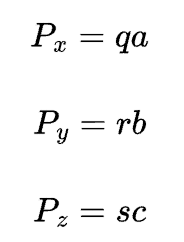
directional indices are computed in terms of differences between vector head and tail coordinates
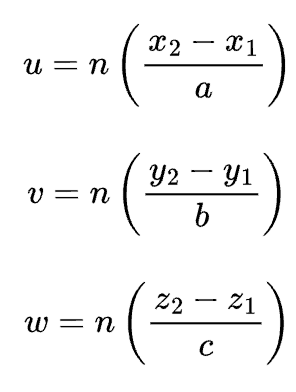
Crystallographic planes
for hexagonal unit cells, a four-index scheme for both directions and planes is found to be more convenient. Directions may be determined using
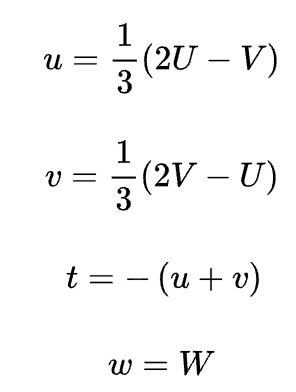
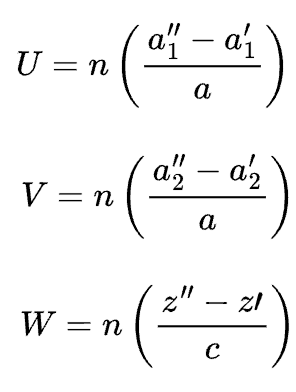
Linear and planar densities
crystallographic directional and planar equivalencies are related to atomic linear and planar densities, respectively
for a given crystal structure, planes having identical atomic packing yet different Miller indices belong to the same family
Close-packed crystal structures
both FCC and HCP crystal structures may be generated by the stacking of close-packed planes of atoms on top of one another. with this scheme A, B, and C denote possible atom positions on a close-packed plane
the stacking sequence for HCP is ABABAB…
the stacking sequence for FCC is ABCABCABC…
Single crystals
single crystals are materials in which the atomic order extends uninterrupted over the entirety of the specimen; under some circumstances, single crystals may have flat faces and regular geometric shapes
Polycrystalline materials
the vast majority of crystalline solids
composed of many small crystals or grains having different crystallographic orientations
a grain boundary is the boundary region separating two grains where there is some atomic mismatch
Anisotropy
anisotropy is the directionality dependence of properties
for isotropic materials, properties are independent of the direction of measurement
X-ray diffraction: determination of crystal structures
X-ray diffractometry is used for crystal structure and interplanar spacing determinations. A beam of x-rays directed on a crystalline material may experience diffraction (constructive interference) as a result of its interaction with a series of parallel atomic planes.
Bragg's law specifies the condition for diffraction of x-rays
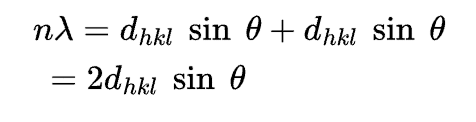
Noncrystalline solids
Noncrystalline solid materials lack a systematic and regular arrangement of atoms or ions over relatively large distances (on an atomic scale). Sometimes the term amorphous is also used to describe these materials.
List of symbols