ENANTIOMERS
Stereochemistry - three-dimensional arrangement of atoms (groups) in space
Stereoisomers: molecules with the same connectivity but different arrangement of atoms (groups) in space
Molecular Chirality: Enantiomers
Enantiomers: non-superimposable mirror image isomers.
Enantiomers are related to each other much like a right hand is related to a left hand
Enantiomers have identical physical properties, i.e., bp, mp, etc.
Chirality (from the Greek word for hand). Enantiomers are said to be chiral.
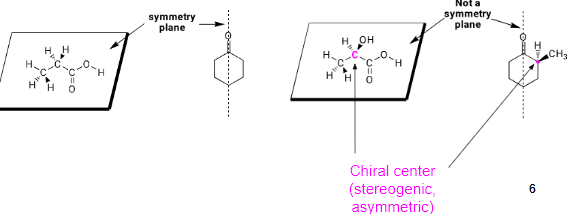
Molecules are not chiral if they contain a plane of symmetry: a plane that cuts a molecule in half so that one half is the mirror image of the other half. Molecules (or objects) that possess a mirror plane of symmetry are superimposable on their mirror image and are termed achiral.
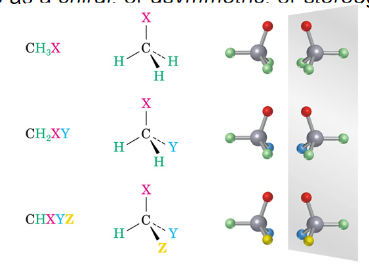
The Chirality Center
A molecule containing a carbon with four different groups results in a chiral molecule, and the carbon is referred to as a chiral, or asymmetric, or stereogenic center.
Enantiomers: non-superimposable mirror image isomers

Symmetry in Achiral Structures - Any molecule with a plane of symmetry or a center of symmetry must be achiral.
Optical Activity - molecules enriched in an enantiomer will rotate plane polarized light are said to be optically active. The optical rotation is dependent upon the substance, the concentration, the path length through the sample, and the wavelength of light.
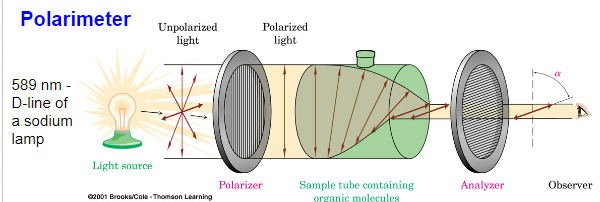
Plane polarized light: light that oscillates in only one plane
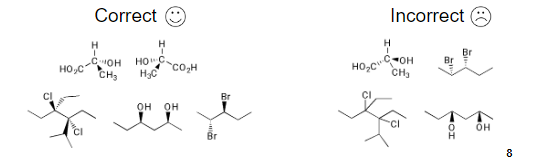
LINEAR ALKANES: You should draw the carbon backbone in the plane of the paper, and draw substituents either coming towards you (with wedges) or going away from you (with dashes). Note that each carbon should look like a tetrahedron.
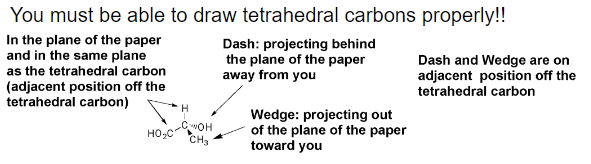
Fischer Projections - representation of a three-dimensional molecule as a flat structure. A tetrahedral carbon is represented by two crossed lines:
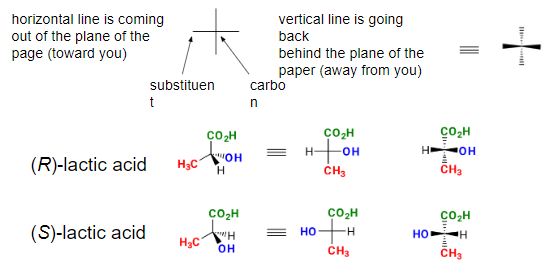
Properties of Enantiomers -In general, enantiomers have the same physical properties (bp, mp, density, etc). Enantiomers will rotate plane polarized light the same magnitude (α) but in opposite directions (+ or -).
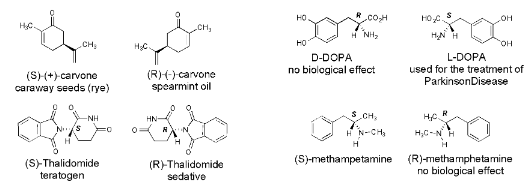
Enantiomers can have significantly different biological properties
Chiral molecules with two chirality centers
What is the relationship between these stereoisomers?
(2R,3R) and (2S,3S) are enantiomers
(2R,3S) and (2S,3R) are enantiomers
Diastereomers: non-mirror image stereoisomers. Occurs when more than one chiral centers are present in a molecule.
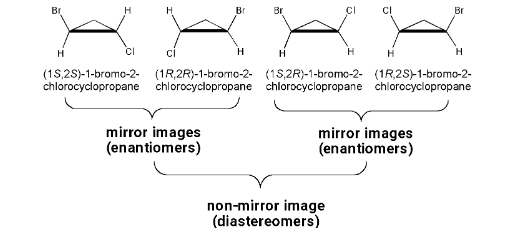
Achiral Molecules with Two Chirality Centers
Meso: molecules that contain chiral atoms but are achiral because they also possess a plane of symmetry.
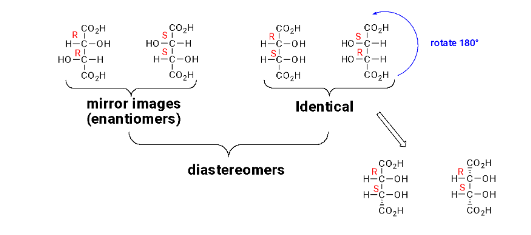
meso tartaric acid: The groups on the top carbon reflect (through the symmetry plane) onto the groups on the bottom carbon.
A Brief Review of Isomerism
Isomers: compounds with the same chemical formula, but different arrangement of atoms
Constitutional isomer: have different connectivities (not limited to alkanes)
Stereoisomers: Atoms connected in the same way, but different
three-dimensional arrangement of atoms or groups (topology)
enantiomers: non-superimposable mirror image isomers
diastereomers: non-superimposable, non-mirror image isomer (more than one chiral center.
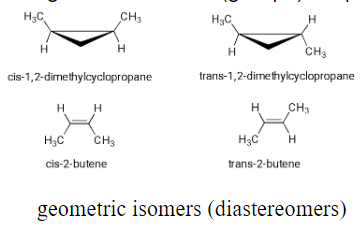
geometric isomers (diastereomers): E / Z alkene isomers