Pratyay Jakkula - Study Guide - 11474557
Name: Block: _
Heat Review/Study Guide
Define the following terms:
- Heat: the transfer of energy between objects at different temperatures
- Temperature:
- Thermal energy:
Temperature scales:
- What is the SI scale?
- What is the lowest possible temperature and why?
- Why does temperature remain constant during a phase change like melting?
- What temperature does water boil at?
- At which temperature does water freeze?
States of matter:
- Which state of matter has the least amount of energy?
- Which state of matter has the most?
- What does it mean to melt? Be specific! Describe what is happening to the particles
- Look at the following graphs, label the phase change occurring during them.
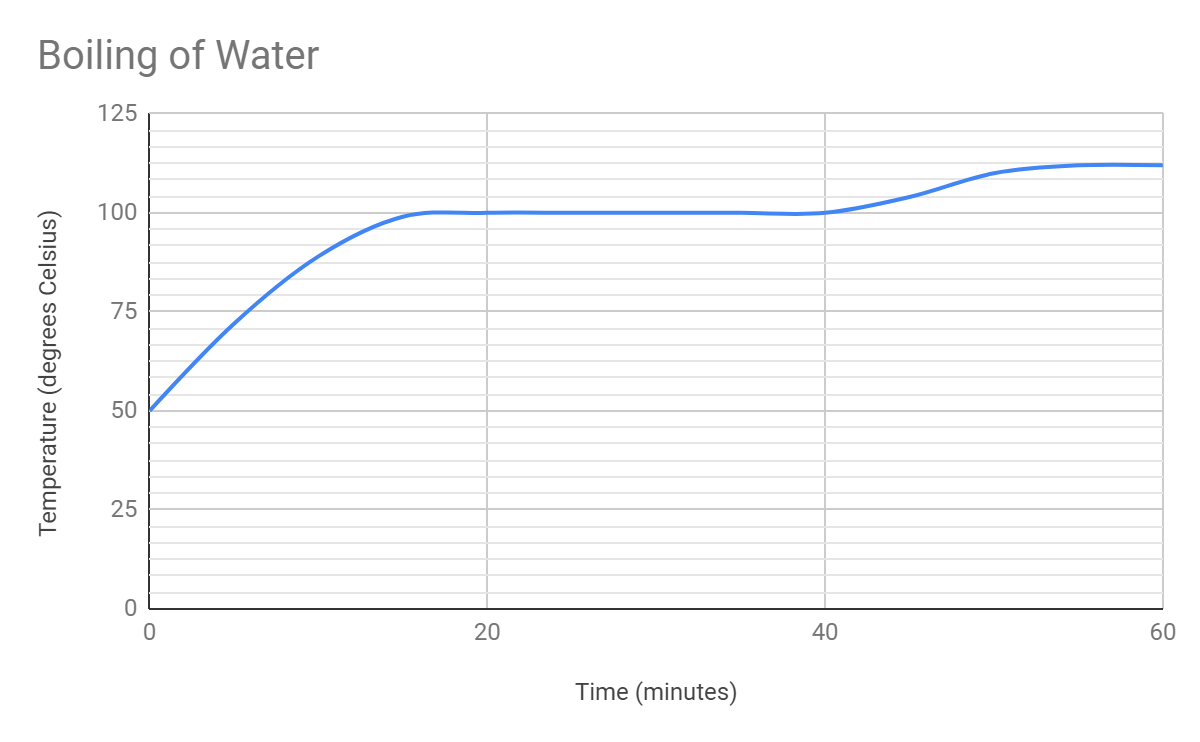 | 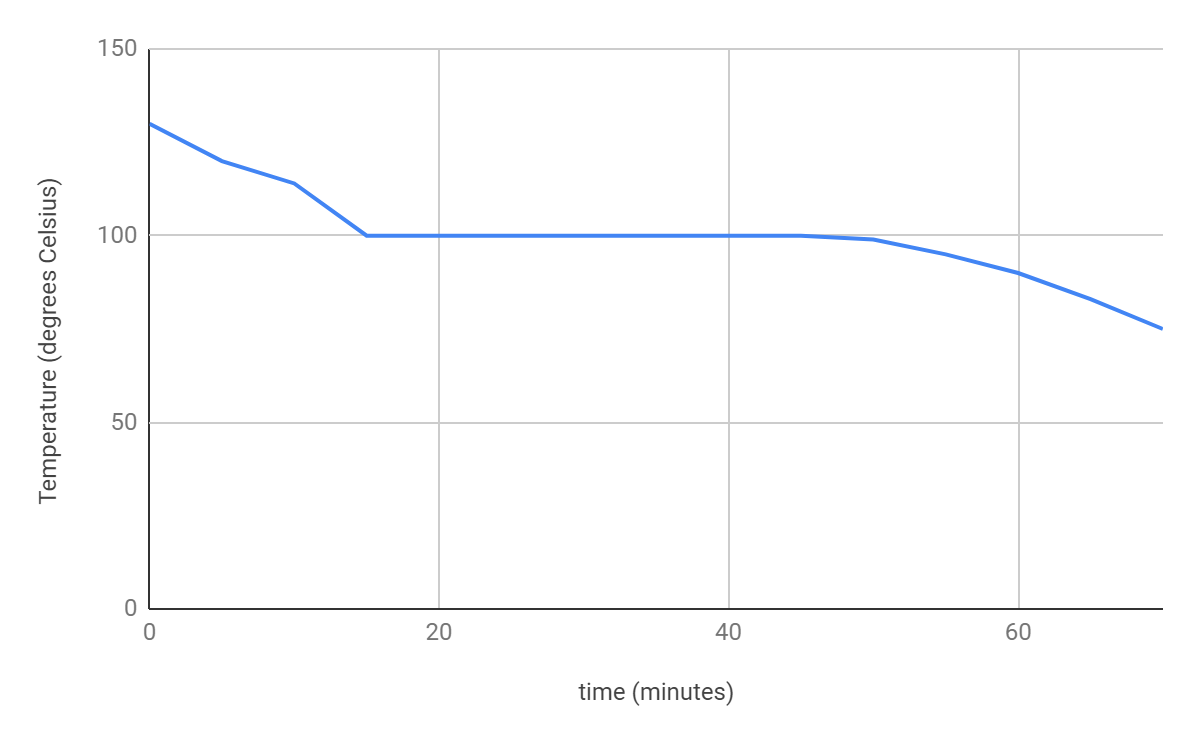 |
|---|
- Where on the graphs is there no change in temperature and why? Be specific! Describe what is happening to the particles.
Thermal energy:
- What is the SI unit of thermal energy?
- Which has more thermal energy?
 |  |
|---|---|
 |  |
- What changes the amount of thermal energy?
Heat:
Define
- Conduction
- Convection
- Radiation
Label the types of heat
 | Why: |  | why: |
|---|---|---|---|
 | Why: | 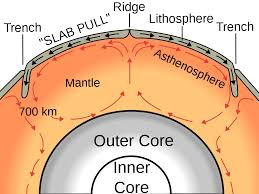 | Why: |