CELL BIO UNIT 2 NOTES
Chapter 14
Oxidative phosphorylation - ADP + Pi ->ATP THIS IS INNER MEMBRANE
Electron transport equation- NADH + ½ O2+H+->NAD+ +H2O NET OF INNER TRANSPORT
Biological oxidation is important so humans don’t combust spon
-famously and the energy in the bonds are in small dosages
O2 is important for biological oxidation so there is a place for
Energy to go
Citric acid cycle> NADH dehydrogenase>Quinone>cytocromeC(REDUCED)>cytocrome C oxidase(OXIDIZE)
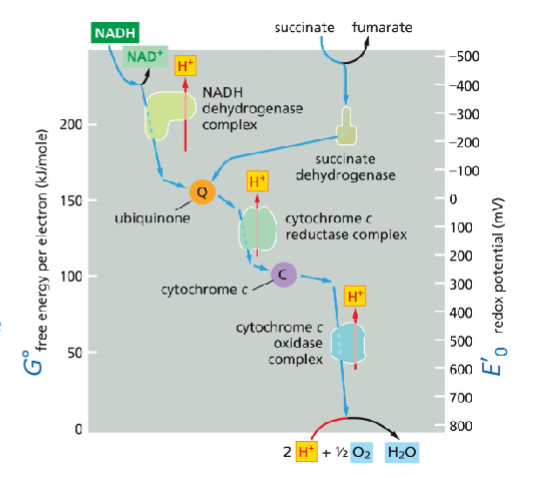 MEMORIZE THIS
MEMORIZE THIS
Chapter 14BCONT
First is reduced and then oxidized
redox(mV) to free energy(kJ/mole)
E’delta=mV, G0=kJ/mole
The more positive redox potential the stronger acceptor more NEGATIVE BETTER DONOR
n=#ofelectrons
CAVEAT redox potential can be influenced by redox conc3entration
Heme group attached to c cytocrome
Copper is critical to complex 4
Iron sulfur cluster
Q(noOH), QH(1OH), QH2(2OH)
None, semi, all
MATRIX ARM -electron transport
MEMBRANE ARM-proton pumping
Proton highways are good and 40X fast but its non-selective , we dont know if its unidirectional
When 2 H+ joins Q it becomes QH2
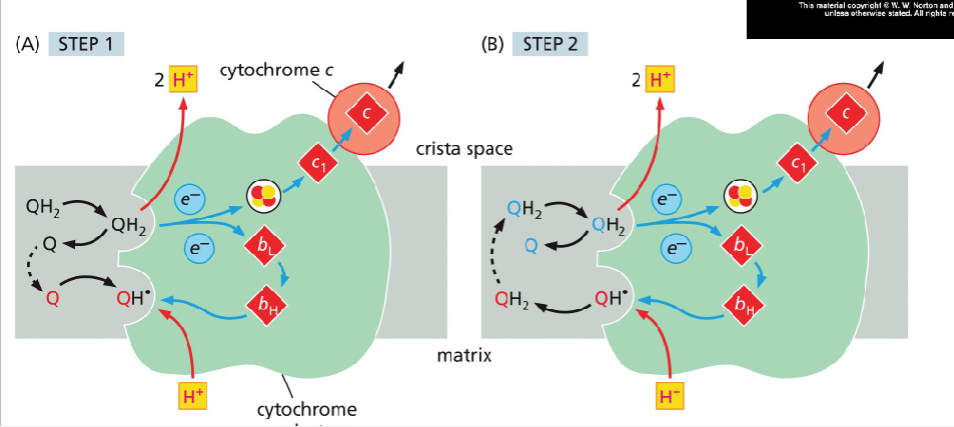
Through cytocrome reductase QH2>iron sulfur cluster>cytocrome b>hemebh>heme bL>cytocrome c1>cytocrome c
none>semi then semi>all
Cytocrome C passes through cu and then heme
iron/sulfur>su>heme
Cytocreme C can indicate cell death
FAD from succinate dehydrogenase donates to Q
CH 14C
1 glucose > 2 pyruvate+2NADH(1.5ATP)+2ATP GLYCOLYSIS
2pyruvate>2acetyl coA+2NADH(2.5 ATP per) PYRUVATE DEHYDROGENASE
2acetyl CoA>6NADH+2FADH2+2GTP CAC
2pyruvate > 8NADH+2FADH2 (1.5 ATP)+2GTP NET MITOCONDRIA
1 palmitoyl CoA-> 8Acetyl CoA +7NADH +7 FADH2 FATTY ACID OXIDATION
8 Acetyl CoA.>24 NADH + 8 FADH2 +8 GTP CAC
1 palmitoyl CoA >31 NADH +15FADH2 +8 GTP NET RESULT MITOCONDRIA
NADH has 2 values because LOCATION CHANGES
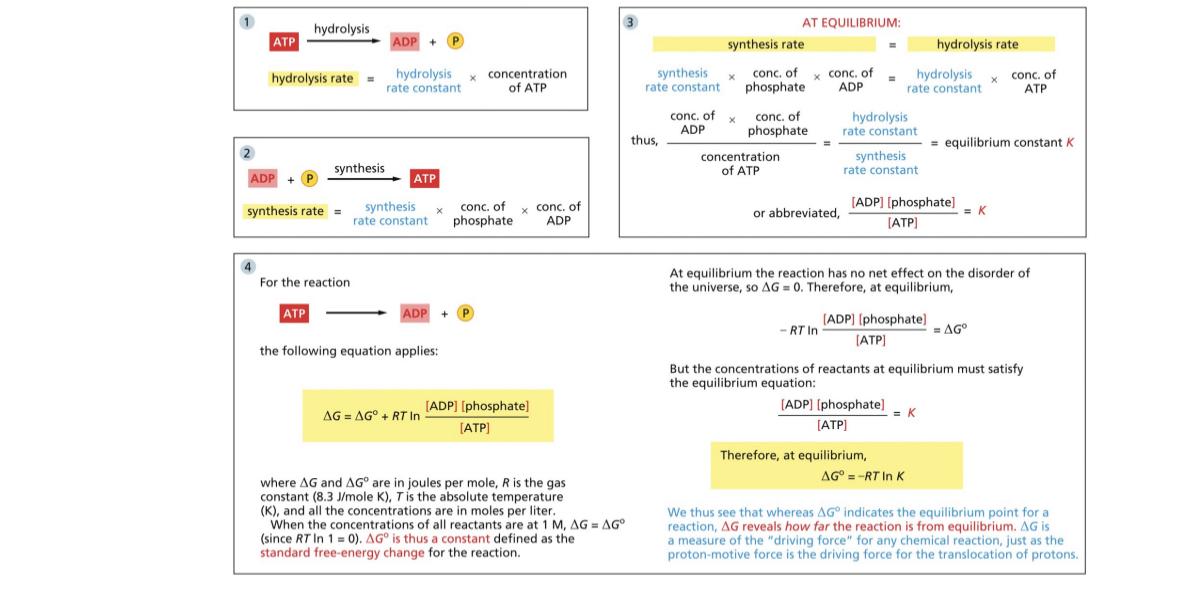
ATP hydrolysis is -30.5kJ/mole
-54kJ is Gdelta or 1ATP
Nadh to oxygen is spontaneous
14 protons in total
Only 61.4% of energy reaches the next stage
- delta G is coupled with positive and for example NADH oxidation and creation of the proton gradient are energetically coupled
Diffusion of the proton gradient-and generation of atp synthase+
Long arm is stator in ATP synthase
There is a rotor stalk
As the protons move they loose their negative and is pushed into the matrix
The stator holds F1 stable so the F1 can catalyzize atp synthesis
Each bump in the images is created by hairpin protien (F0)
13-15 hairpins but can be as low as 8-9 or 21-23(F0)
You need 1 proton for every hairpin
The difference in charge drives the rotor around
There are dimers that stabilize the atp syntheses
There is a sink for atp synthase
8000 revolutions per minute,3 atp per turn
ADP/ATP cotransporter
The inter membrane space will be the more positive side
ATP being negative drives directionality
Bacteria use proton to pump nutrients into the cell and ATP
In anerobic atp pumps and Na but no gradient is established
CHAPTER 14 D
Stroma = matrix
Thylakoids= cristae
ATP in plants is produced in the stroma and consumed
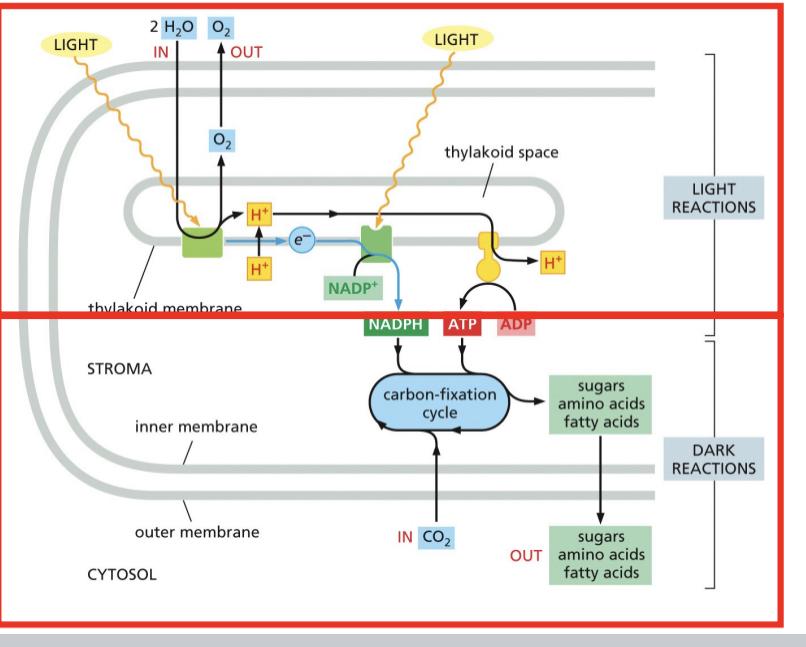
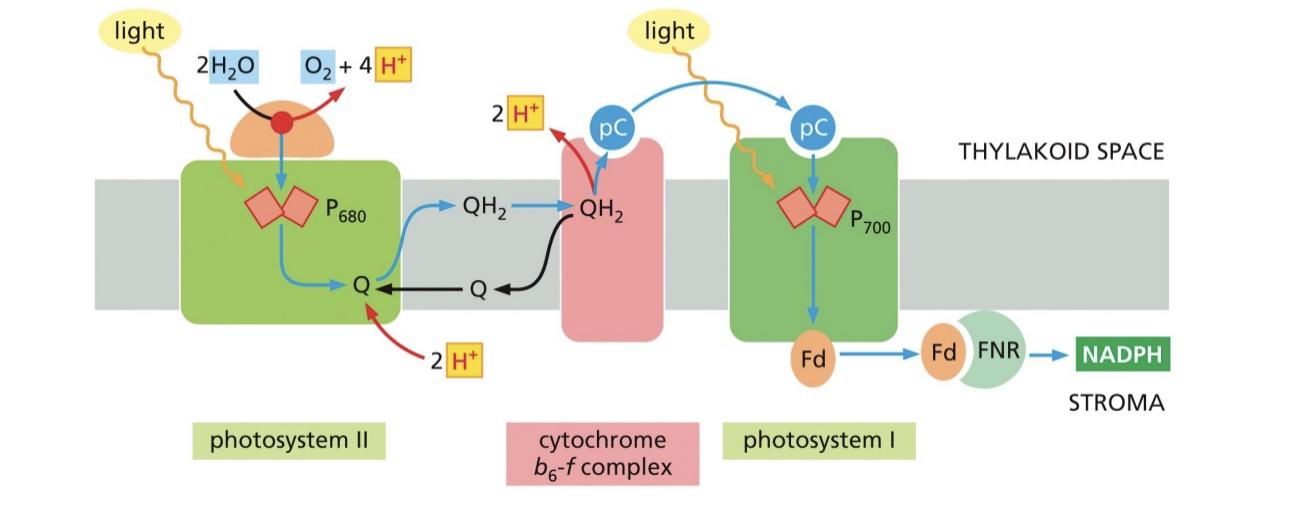
The high level electrons get excited and water replaces those electrons
Dark reactions are dependent on the products of light
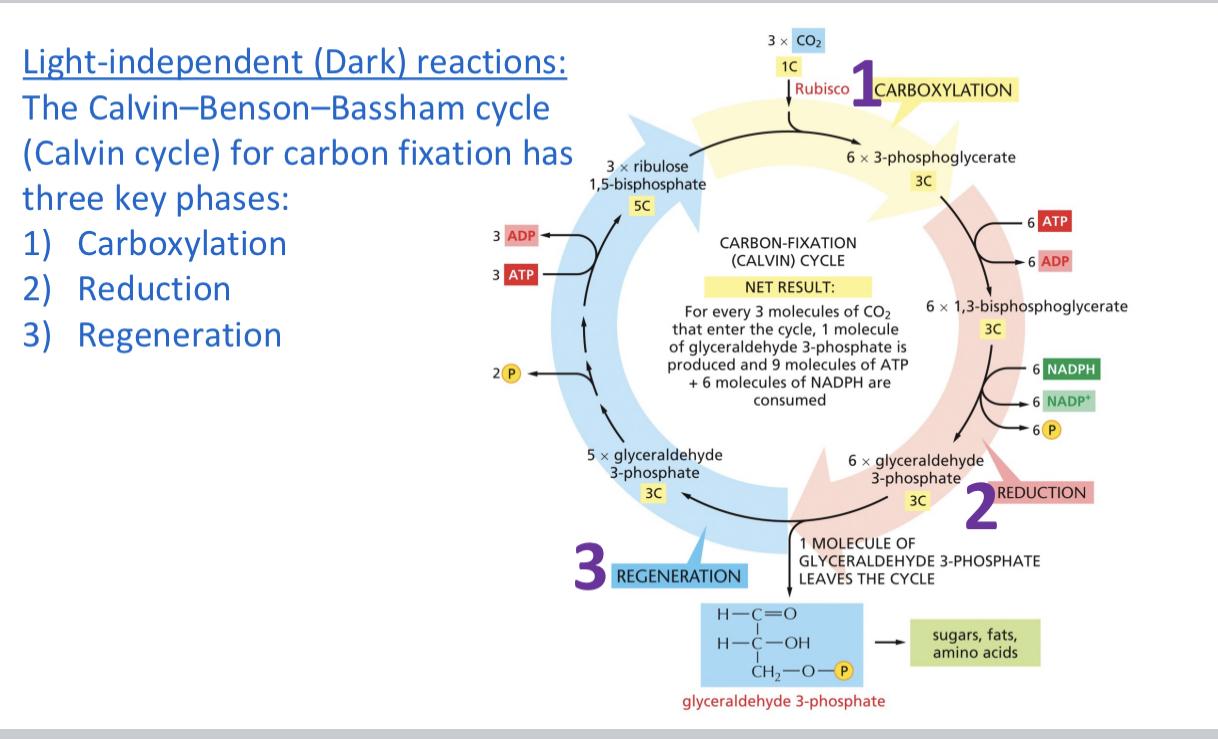
Carboxylation, take the product 5C +1C=6C/2=3C molecules
Rubisco fixes the carbon
Reduction is gain of electrons and remodeling of bonds 6 3 Carbon molecules NADPH is in the bond energy , 5 continue because we keep one to break down
REGENERATION 5 * 3C +ATP from light
Carboxylation is CO2+ribulose 1,biphosphate>w/rubisco intermediate +H2O2 molecules of 3-phosphoglycerate
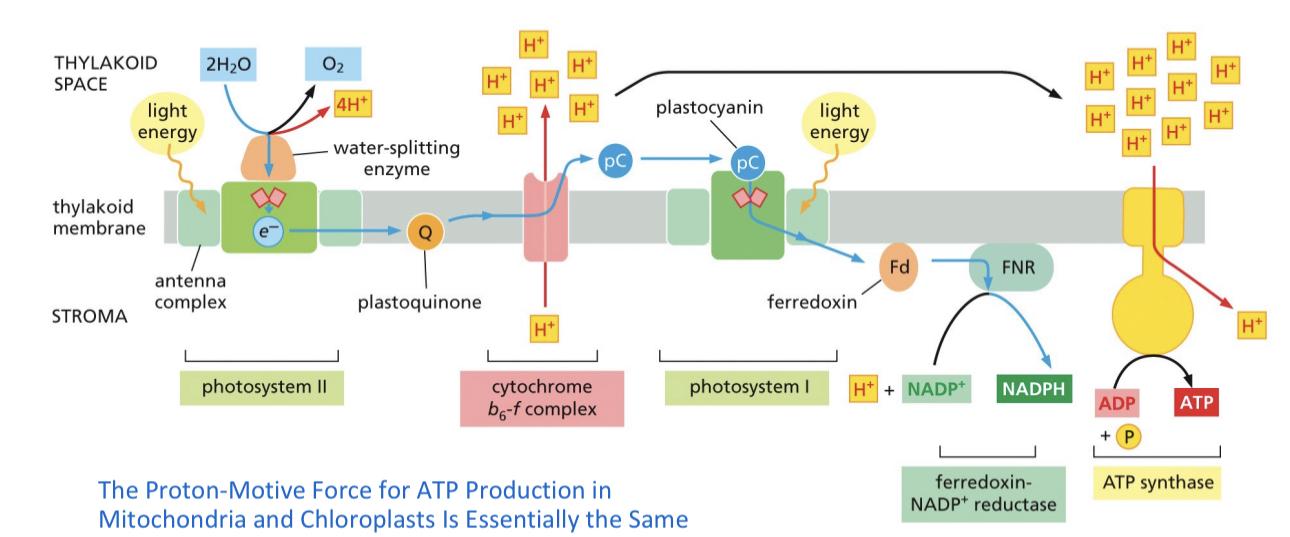
Review the photo system video
100 per second
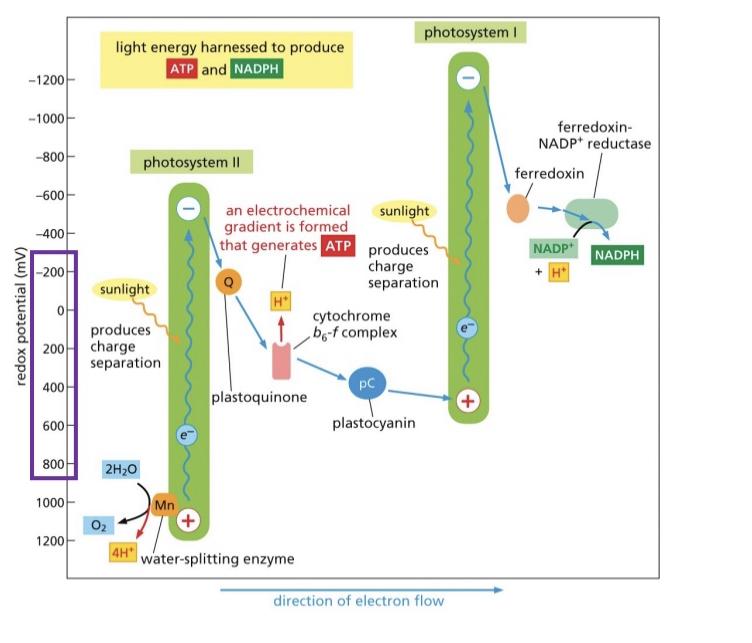
Mn cluster splits the water for electrons to be excited into the zscheme
Photo system 2 only has one pathway that’s used rapid
The alpha rotor has 12 subunits=12 - charged residues
Every time a proton enters the hairpin is moved one over
How many protons are needed to turn =how many subunits there are
Every 12 protons 3 ATP
Ancient origin ATP synthesis (used originally for protons not ATP) first then ETC and then they’d are combined
Started from ancestral fermenting minimal atp and H2S synthesis’s
Purple non sulfur bacteria used something like cytochrome c but it wasn’t
CHAPTER 16A
Freeway system made out of ants
Cytoskeleton maintains org,cell structure , cell shape changes,cell movement, and intracellular trafficking of vesicles/organelles, cell division
Cytoskeleton react in response to a lot of signals
Repolarize-rebuild cell skeleton
Actin and microtubules are dynamic to refunctionung
All filaments need 1000 monomeric subunits and though they are structurally simple prokaryotes have ace steal versions of these filaments
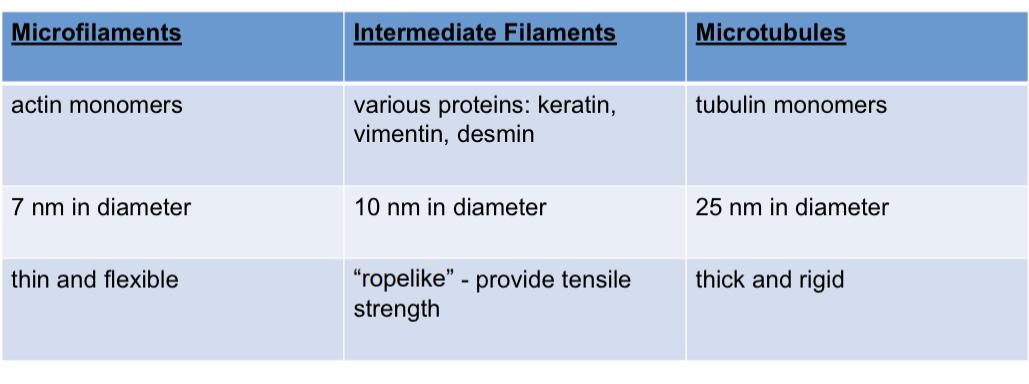
Actin fibers can be like a fishing line, very versatile
Microtubules have + or - ends
All of these filaments can interact to make and maintain a mature cell cytoskeleton
Intermediate filaments are made of a-helical region its coiled together until dimer becomes a Teramer and it is no longer parallel
This is a really strong 32 protein chains and it is a very strong
Mesenchymal cells muscle and keratin
Nuclear laminate give tremendous structural support unless it’s phosphorylated and it falls apart
Epidermal IF keratin filaments connect through desmosomes or hemidesmosomes
EBS rapid blistering due to tetramers not being able to form into protofilaments
Axons IFs-
Actin filaments, most abundant proteins in animals, all cells need it, 7nm , used as a reference control in everything and thin mesh work
Repeat overly short lived , creates striated muscles
Dynamic burst of polymerazation makes the cell move really quickly
G-Actin -globular actin is a mono subunit
It is a spherical contact had atp Imbedded inside the gactin
Factin- there is a + and - end
Suitable pool of charged g-actin ATP
Add more readily to the + end
You cannot depolymerize from the middle
Polymerization happens in one configuration
Origin of actin actin and myosin bonds and
Myosin creates a barb and the plus end is more barbed
The process of polymerizing 1.lag phase2.growth phase3.equilibrium phase lots of gactin to factin
Lag phase - nucleation it’s rryl long
The growth phase occusd as a monomer add to the exposed ends of the growing filaments,causing filament elongation
If a gactin needs to be added then it needs to be removed, balanced
We run out of free subunits and it hits an equilibrium
Disassembly is more favorable at the - end
Once they are in the polymer there is no difference in the - and + surface
The plus end is more stable because they ATP binds initially (glue) that does not happen to the negative end
If depolymerazes because of a time differential
Critical concentration is higher for the ATP bound + side then the ADP bound - side
Hydrolysis is not nessecarg
Treadmilling when the actin filaments results when assembly at the plus end is concomitant with depolymerization at the minus end
CHAPTER 16 B
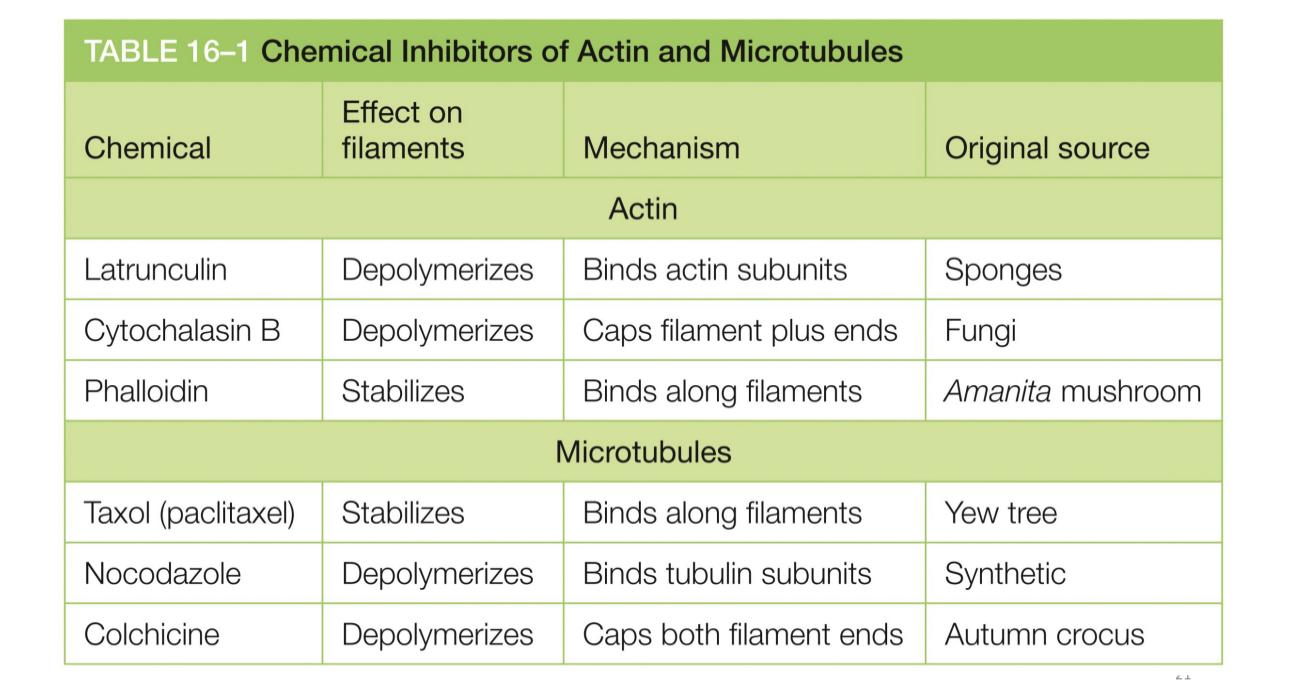
Arp2 and 3 very actin related
Arp3/2 bind to NPF and clicks both into a active state so it can bind to a uncleared actin filament (ON MINUS END)
ARPS kind of glue onto the edge (70° angle) these make branched formations
ADP it’s pushed into the NPF and the actins dissasociatw.
Profilin is opposed by thyomisin
Forms clamp the actin and myosin to the membrane (associated with the plus end)
Uniform Z disks lead to striating
Tropomodilin and CapZ prevent polymerization and depolarizing
We can use coffilin to break off actin filament ends(induces severe twisting)
Creates new ends to deassamble more rapidly
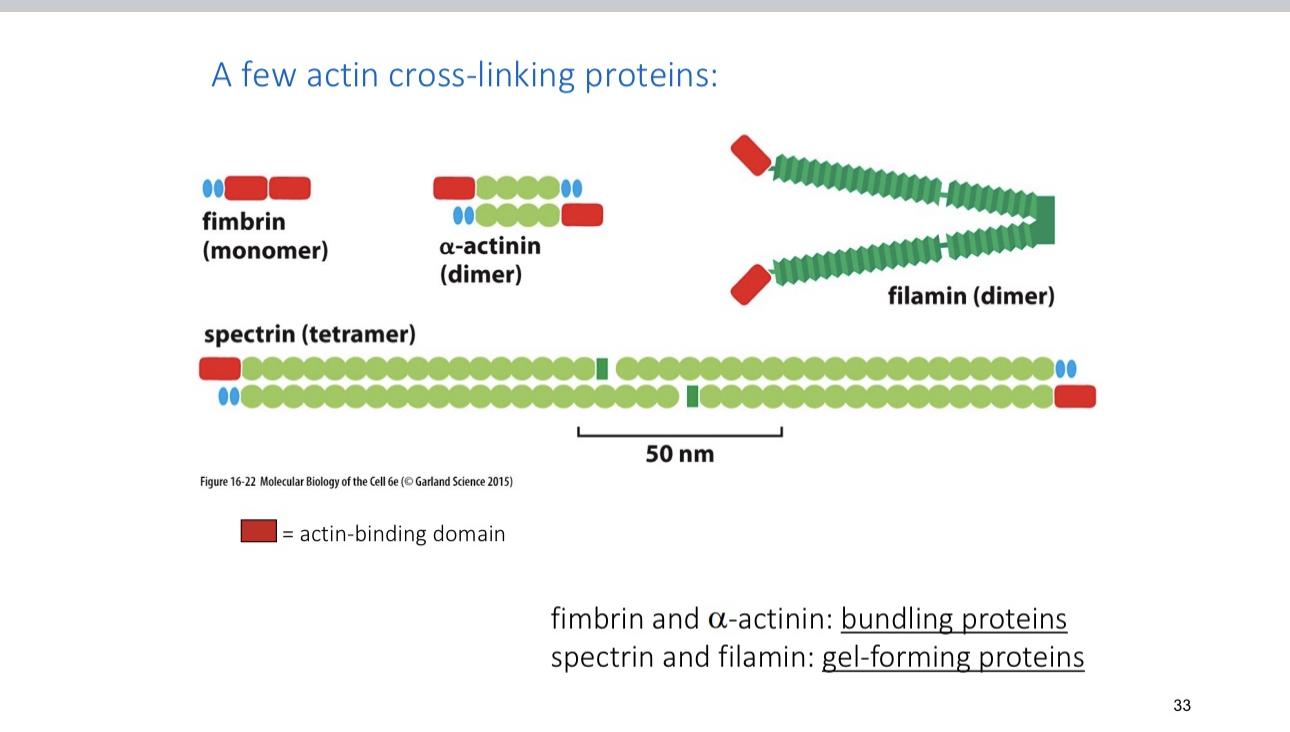
Filament is gel forming and brain relies on it
I. Fibrin it’s exclusively structural
Periventrical heterotrophia-seisures
Myosin a ton binding protien
Listeria bacteria-pathogenic food poisoning coops actin array and pull the subunits to breaking
You can generate force by the actin by densly pushing things to the front
Focal adhesions via integrins to move the leading edge
All of the cell Mobility is myosin dependent
Cell moves like this
Coffilin will twist the actin filaments to have Arp fill in to make it very large
Amobeid cell membrane is facilitated by external sensing and feedback, they hunt for pathogens and commit eating so it can use the actins from them
Ratchet is used to describe the actin filaments moving forward and not back
Profilin recruits actins
Capping protien binds to plus end to stop it Arp is the branch
These factor give polarity and allow the cell to move
A lot of feedback crll regulation
Myosin is an allosteric binding protien that depends on polarity and atp Adele activity, + end directed, actin dependent
Myosin and actin work together to perform cellular functions: vesicle translocation,cytoplasmic streaming,cell locomotion, and muscle contraction
Allosteric-protien with a binding site so the structure can conform to it
Atp and ADP stay in their pocket tnad it effect the relative shape
Myosin is highly concerned
2myosinnprotiens will be interwoven
Myosin 1-the bilayer is attaching to the cortical actin to hydrolyze atp
Myosin 5- the carbon binding protien da drag and grab vesicles by “walking” and it has a 35nm distance(draw this )
Myosin 2- has 2 heavy subunits with a coil coil terminus
The neck regions diversify the force
These are regulated , from the center there is a bare zone
50-60 myosin heads
CHAPTER 19
Cadenerin based protiens respond to adherin junctions
How one cell is connected to another
Cells anchor to other junctions based off cadaherins
High calcium is needed for cadaherims to link and then anchor to the cytoskeleton
Under tension protiens are extended to reduce stress on the molecule
The actin support cell connections, important and weak, useless without adherin junction
Adherin junction can generate force and bend and pull tubes out
Desmosomes cell cell anchoring junctions they are embedded within the plasma membrane with
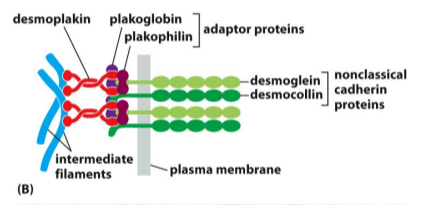
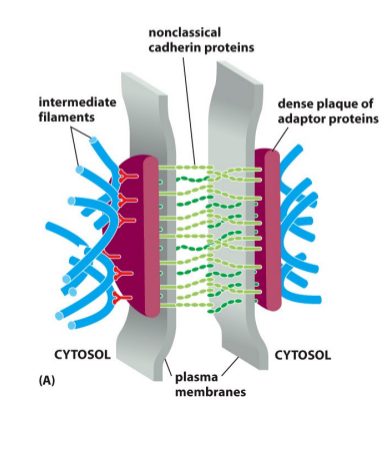
Integrins is like cadaherins but it binds to the extracellular material
Tight Junction- elaborate array of protien, function is to seperate spaces that are created by a layer of cells, separates interstitial and blood stream(Tupperware lid) creates a seal
Series of protiens stitched together (clodins and aculin ) with lateral attachments across
Channel forming junctions or gap junctions- connects cytosol , chemical synapses, only 1000 daltons can pass small ions and molecules can pass
Gap junctions that are small stack together
The ECM is diverse and complicated mixture, its secreted and it can be structural or space filling,
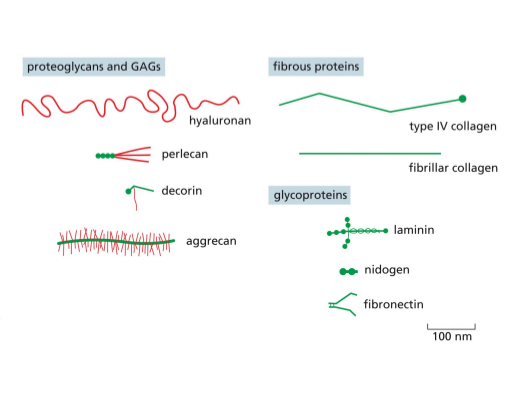
Makes you durable and promotes compression resistance
Fibrous protiens provide tensile resistance
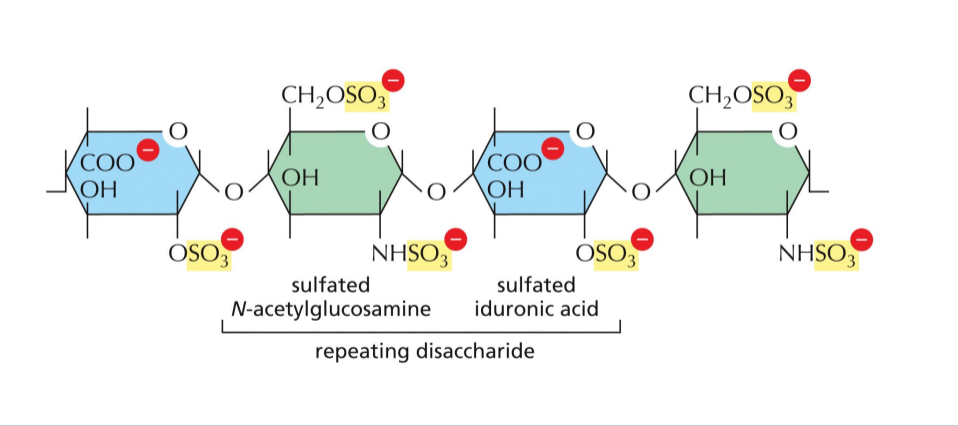
Hyluranan forms the backbone for bigger complex of things like heparin that ca. dock on the hyluran complex
Collagen- an example of a fibrous protien that can provided tensile strength via GAG and it’s usually made of a chain of amino acid, fhe partner chains are woven together
Hydroxylation gives collagen distinctive folding patterns.
Ex bone defects,dwarfism, nasal laminate will fail in the kidenehs , and all sorts of problems that come from a lack of calllahdn
Collagen is dependent on vitamin C as a cofactor
Elastin- forms cross links and are coiled up to allow streachimess and relaxation and prevents the other end without tearing ping or ripping
Finromectim and other multi adhesive protiens all of domains can be switched out via alternitive splicing
Fibromectins bind integrins
Arg protiens allow the integrity protiens allow the cell being knit into the and then show it to. E
Basal lamina is exclusive to the epithelial cells
The key assembly factor that is growing into the cell did 3 rd protien call dystroglucan,indigenous,,perfect
Integrity bind the cell to the extracellular ,atria
Connected to cell with intermembrane filaments, and anchoring junctions
Inactive integrity- makes internal and external signals on the alpha and beta site inactive anchoring
Lateral cross linking protiens mediate binding