The Atom
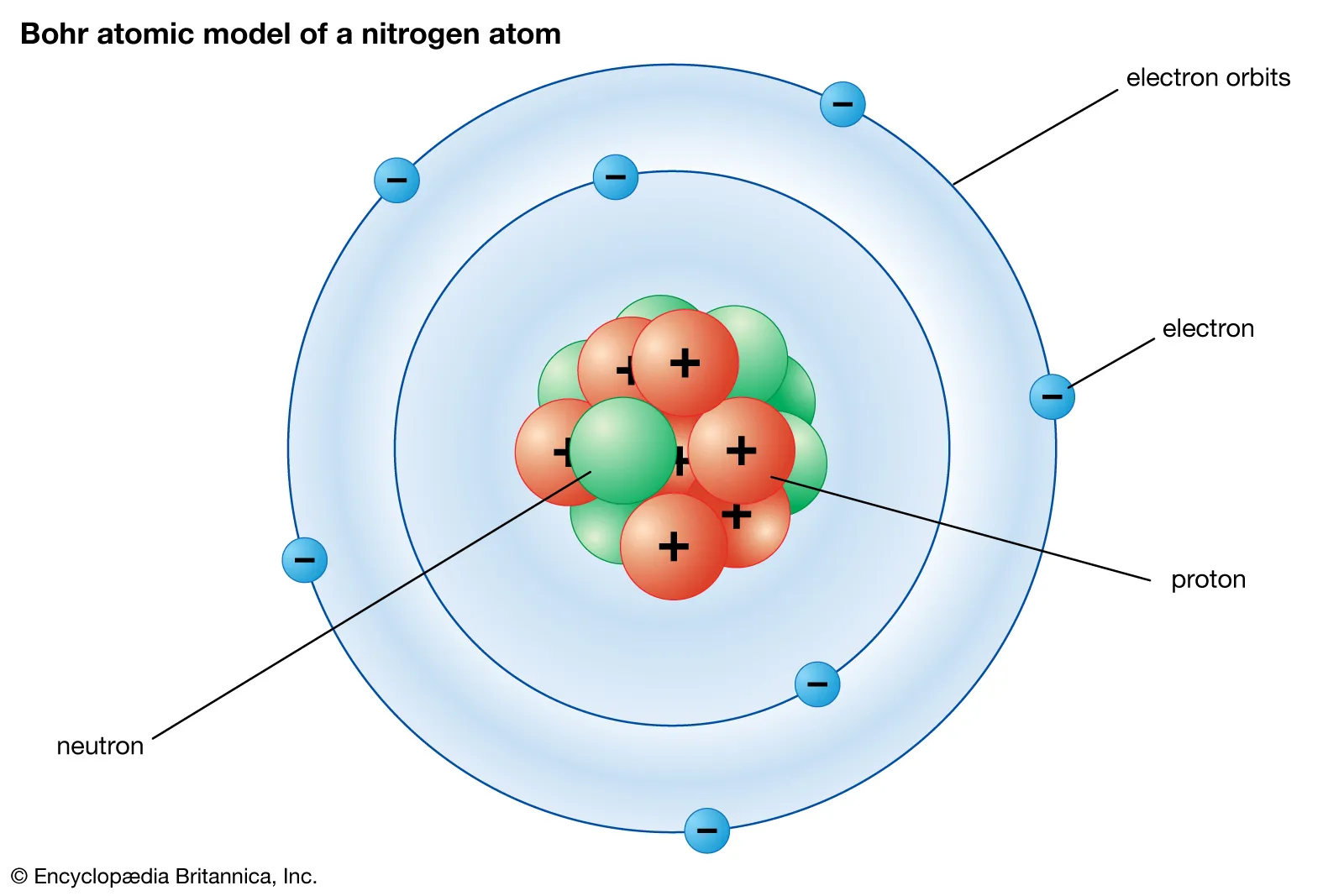
Atoms: The basic building blocks of matter, comprised of protons, neutrons, and electrons.
Protons: Positively charged particle in the nucleus (TEST!!!)
Neutron: Neutral particle in the nucleus (TEST!!!)
Electron: Negatively charged particle that orbits in outer shells around the nucleus. (TEST!!!)
Helpful videos:
History of the Atom
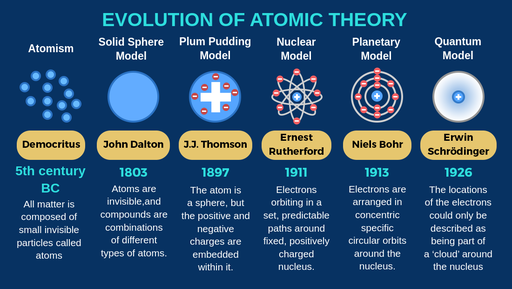
Democritus’ atomic theory: Proposed that matter is composed of indivisible atoms, which combine in various ways to form different substances.
Dalton’s atomic theory (TEST!!!): All matter is composed of tiny, indivisible particles called atoms, where atoms of the same element are identical, atoms of different elements have different properties, and atoms combine in simple whole number ratios.
Thomson’s atomic theory: An atom is a sphere of positively charged matter with negatively charged electrons embedded within it (Plum Pudding Model)
Rutherfords atomic theory (TEST!!!): Proposes that an atom consists of a tiny, dense, positively charged center called the nucleus, surrounded by negatively charged electrons orbiting in empty space, with the nucleus containing nearly all of the atom’s mass. Also known as the nuclear model.
Bohr’s atomic theory (TEST!!!): Explained that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy.