Note
0.0(0)
Chat with Kai
Explore Top Notes Note
Note Studied by 2 people
Studied by 2 people Note
Note Studied by 47 people
Studied by 47 people Note
Note Studied by 11 people
Studied by 11 people Note
Note Studied by 3 people
Studied by 3 people Note
Note Studied by 72 people
Studied by 72 people Note
Note Studied by 16249 people
Studied by 16249 people
VTV casus 5
5.0(1)
Ch. 1, Lesson 1 -- Being an American
5.0(1)
J 201 Midterm
5.0(1)
Exploring Structures Using Waves
5.0(1)
Macbeth: Macbeth Key Quotations
4.5(2)
Big Idea 2: Data
4.8(51)
Titration and Buffers Critical Thinking Questions
Why is the graph of the titration curve so steep as the equivalence point is approached?
no more weak acid to neutralize strong base → any addition of the base will lead to rapid increase in pH
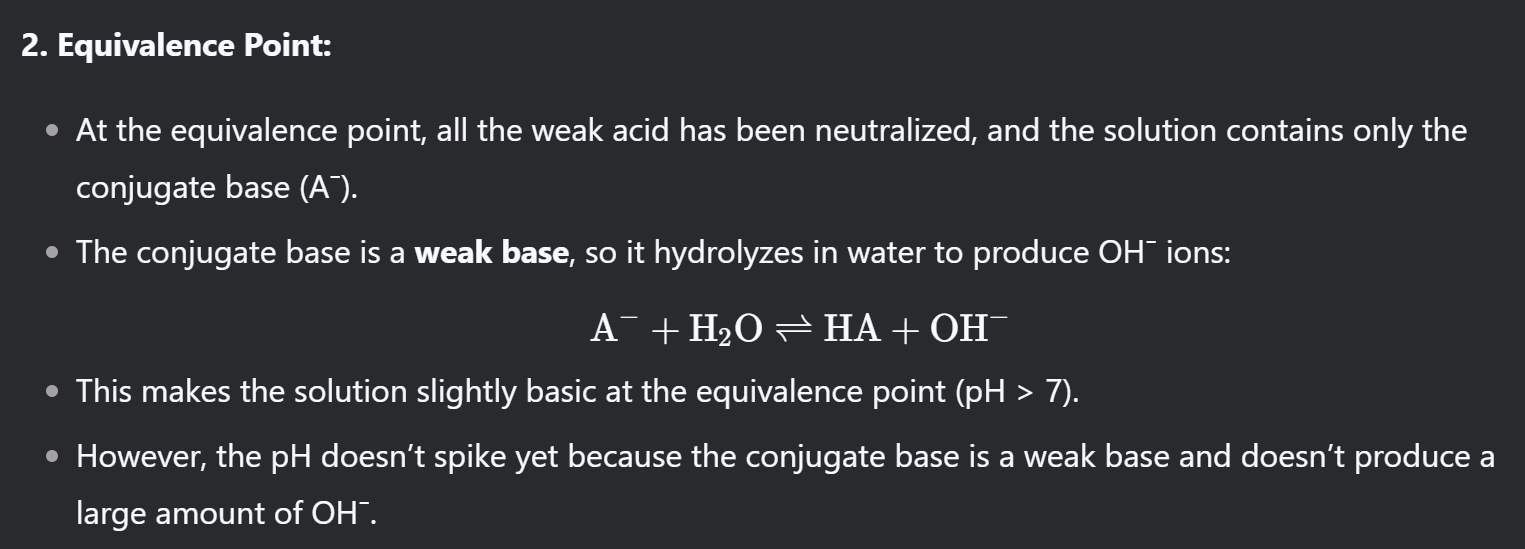
When titrating a weak acid with a strong base, why does the graph rise so slowly before the vertical section around the equivalence point?
Note
0.0(0)
Chat with Kai
Explore Top Notes Note
Note Studied by 2 people
Studied by 2 people Note
Note Studied by 47 people
Studied by 47 people Note
Note Studied by 11 people
Studied by 11 people Note
Note Studied by 3 people
Studied by 3 people Note
Note Studied by 72 people
Studied by 72 people Note
Note Studied by 16249 people
Studied by 16249 people
VTV casus 5
5.0(1)
Ch. 1, Lesson 1 -- Being an American
5.0(1)
J 201 Midterm
5.0(1)
Exploring Structures Using Waves
5.0(1)
Macbeth: Macbeth Key Quotations
4.5(2)
Big Idea 2: Data
4.8(51)
