PY 131 Chapter 17: Phase Changes
Phases
Most substances can exist in different states e.g. as a solid or liquid, depending upon the temperature and pressure.
A figure showing the state as a function of T and P is called a phase diagram.

Some substances, e.g. hydrogen, have many more phases so their phase diagrams can get a bit complicated.

Phase Changes (Transitions)
- A substance can change its phase, from e.g. solid to liquid or solid to gas.
- This can be accomplished by heating or cooling it (changing its temperature) or squeezing it (changes in pressure).
- For some substances (e.g. mixtures) phase changes can occur by changing some other property (e.g. concentration)
Melting/Freezing
- Melting is the phase change a substance undergoes when it transitions from a solid to a liquid.
- Freezing is the opposite.
- During melting/freezing the temperature of the substance remains constant until all the substance has changed phase.
- Melting/Freezing temperatures or pure substances can be altered by impurities e.g. salt in water.
- Melting is usually accomplished by adding heat.
- When something freezes the heat must be removed
- The heat added to 1 kg of a substance to cause melting is called the latent heat of fusion.
- The energy is used to break interatomic/intermolecular bonds.
- The latent heat of fusion for water is 335,000 J/kg
- When a substance freezes the latent heat of fusion is absorbed by the environment.
- For a few substances such as water, melting/freezing can also be accomplished by applying/removing pressure.
- Water expands upon freezing which is not common.
- Applying pressure to ice increases its density.
- When the density of the liquid phase is reached, the ice melts.
- At 500 atmospheres the melting point of water is -4 °C.
- When the pressure is removed the liquid refreezes into a solid.
- Melting due to applied pressure and refreezing when it is removed is called regulation.
- Regelation is different from surface melting (which makes ice slippery).
- Regelation can occur to the ice under a glacier but making snowballs does not involve regulation.
Boiling/Condensing
The phase change between a liquid and a gas is called boiling.
Condensing is the change from a gas to a liquid.
The boiling point of most liquids can be changed with pressure and with the addition of impurities.
The energy required to change 1 kg of a liquid to a gas is called the latent heat of vaporization.
The latent heat of vaporization for water is a 2,260,000 J/kg
- The latent heat of vaporization for a substance is always much larger than the latent heat of fusion.
When a substance condenses the latent heat is released into the environment.
The temperature versus heating graph for water shows the two phases’ changes.
- It takes more energy to boil 1 kg of water than it does to melt 1 kg of ice and then raise its temperature to 100 °C.

EXAMPLE 1
When rain forms in clouds, the surrounding air is
A. cooled.
==B. warmed.==
C. insulated.
D. thermally conducting.
The condensation of water droplets releases the energy of vaporization
Evaporation
- Evaporation is a different process than boiling.
- During evaporation fast-moving atoms/molecules at a liquid surface escape to a gas phase.
- The loss of above-average energy atoms/molecules lowers the average of the remainder.
- Evaporation thus cools the remaining liquid.
- If the remaining liquid is in thermal contact with another object at higher temperature, heat will be withdrawn from the hotter object.
Dew points
- As the temperature of a mixture of two (or more) gases is reduced, one of the gases may form droplets of its liquid phase.
- For a given mixture of gases, the temperature at which this occurs is called the dew point.
- If the dew point temperature is below the freezing point it is called the frost point.
- A similar effect can occur in mixtures of liquids when one component of the liquid will freeze into its solid phase or the liquid phase separates (cease to mix).
- The temperature at which this occurs is called the cloud point.
- Examples include cooled oils.
Other Phase Changes
- The phase changes from solid to liquid, or liquid to gas (and vice versa) are not the only possibility.
- Other phase changes include:
- from solid to gas (sublimation/deposition)
- from one solid phase to another solid phase,
- from a neutral gas to a plasma (ionization/recombination)
- from magnetized to non-magnetized
- between different molecular structures e.g. molecular hydrogen to atomic hydrogen, O2 to O3
- quantum phase transitions where one phase exhibits quantum behavior e.g. superconductivity, superfluidity, quantum condensation
Sublimation/Deposition
- The phase change from solid to gas is called sublimation.
- Deposition is the change from gas to solid.
- Whether a substance sublimates or melts depends on the (partial) pressure of the gas above the solid.
- The energy required to sublimate 1 kg of a substance is called the ==heat of sublimation==.
- In freeze drying, an object containing water is first frozen in a chamber then the pressure in the container is reduced and the ice sublimates.
Ionization/Recombination
- Charged atoms/molecules are called ions. Electrons that are not bound to an atom/molecule are called free electrons.
- A mixture of free electrons and ions is plasma.
- The removal of electrons from gaseous atoms/molecules is called ionization and is a phase change.
- Ionization is different from the dissociation of e.g. solid NaCl in water.
- The energy required to remove an electron from a single atom/molecule is called the ionization energy.
- Ionization can be accomplished by adding heat but there are other alternatives.
- e.g. shining light, and collisions with energetic particles.
- Like other phase changes, the temperature remains constant during the phase change.
- The ionization temperature of hydrogen gas is ~10,000 K.
- When electrons are captured by ions the process is called recombination.
- Recombination is the opposite of ionization.
- Usually, the energy released by recombination is emitted in the form of light.
Special Points in a Phase Diagram
There are two types of special points in a phase diagram: triple points and critical points.
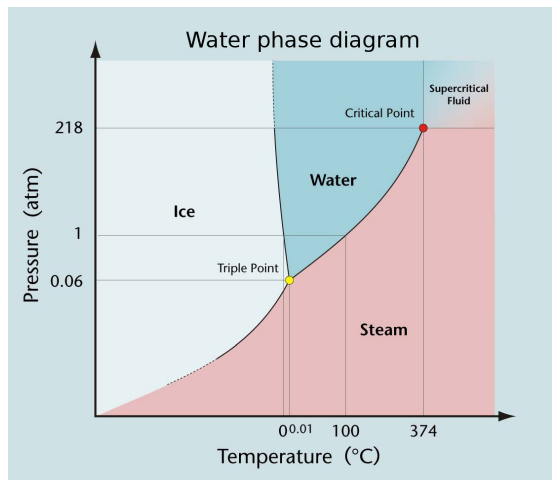
Triple Points
- For every substance that can exist in three phases (e.g. as a solid, liquid and a gas) there is a unique temperature and pressure at which all three phases can coexist.
- This combination of temperature and pressure is called the substance’s ==triple point.==
- You can have triple points between any three phases of the substance e.g. two solid phases and the gas.
- Helium has a triple point between two liquid phases and the gas.
- The triple point of pure water used to be the second fixed point on the Kelvin temperature scale.
- The temperature of the triple point of water was defined to be 273.16 K
Critical Points
- For every substance that can exist as a liquid and a gas, there is a unique temperature and pressure at which the distinction between liquid and gas disappears.
- This combination of temperature and pressure is called the substance’s critical point.
- You can also have critical points between any other two phases of the substance e.g. two solid phases or two liquid phases
- For temperatures above the critical point, gas cannot be liquefied by pressure alone.
- A substance with a temperature and pressure larger than the liquid-vapor critical point is sometimes called a supercritical fluid.
- The critical point of pure water is 373.946 °C and 22,060 kPa.
- Supercritical water can be found around ‘black smokers’, geothermally heated water issuing from vents on the sea floor.
Superheating/Supercooling
- Phase changes don’t occur uniformly in a substance, they are usually patchy e.g. when a liquid boils it forms bubbles.
- Bubbles in a boiling liquid often form around imperfections of some kind which form nucleation sites.
- Bubbles start small and then grow (they expand and the amount of gas in the bubble increases due to evaporation from the bubble surface)
- The gas pressure must overcome the liquid pressure and also overcome the surface tension of the liquid.
- Because a small bubble has a large surface-to-volume ratio, it takes a larger gas pressure to make a small bubble than a big bubble.
- If small bubbles cannot form, the liquid does not undergo the phase transition and can be heated to a temperature higher than the boiling point.
- Such a liquid is called ==superheated.==
- Superheated liquid hydrogen was once used in high-energy particle detectors known as ‘bubble chambers’.
- Particles traveling through the superheated liquid create nucleation sites which then form bubbles.
- Photographs of the bubbles reveals the path of the particle.
- A similar phenomenon can occur when a gas tries to condense into a liquid or when liquids try to freeze into solids.
- Without nucleation sites the gas can be cooled below the boiling temperature or the liquid below its freezing temperature.
- Such a gas / liquid is called ==supercooled.==
- Pure water can be supercooled to −48.3 °C.
- Supercooled carbon dioxide gas was once used in high energy particle detectors known as ‘cloud chambers’.