7.1 Discrete energy and radioactivity
7.1 Discrete energy and radioactivity
Assumption in Physics
While we understand how charges interact through an electromagnetic field, the nature of charges and the distinction between positive and negative charges remain unknown. Despite this lack of clarity, the text suggests that effective theories can still be developed based on these poorly understood concepts.
Electrons in atoms have specific energy levels and can only occupy allowed orbits.
Electrons change energy levels by jumping between orbits.
Allowed energy levels are determined by the wave nature of electrons and quantum mechanics.
Hydrogen atom consists of a proton and an electron bound by electromagnetic force.
Different isotopes of hydrogen exist with varying numbers of neutrons.
A hydrogen atom without an electron becomes a positively charged hydrogen ion.
the planetary model of the atom where electrons orbit the nucleus like planets around the sun. It introduces energy levels in atoms. The table shows energy levels for hydrogen atom:
Energy Level (n) | Energy/eV |
|---|---|
1 | -13.58 |
2 | -3.39 |
3 | -1.51 |
4 | -0.85 |
5 | -0.54 |
Energy levels in atoms are shown diagrammatically in graph below.
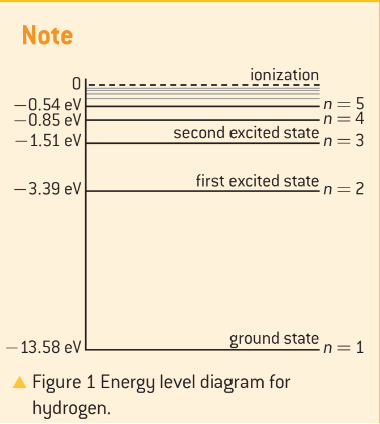
Electrons in different levels have specific energies.
Ground state electron in hydrogen atom has -13.58 eV.
Excited states have higher energy levels.
Energy levels in atoms are quantized with discrete values.
Transitions between Energy Levels in Hydrogen Atom
Electron jumps between energy levels in discrete amounts
Energy gained must be exact for transition
Energy needed for excitation can come from absorbed light
Energy of photon related to frequency and wavelength
Wavelength of radiation calculated for specific energy transition
Nature of Science and Patterns in Physics
The energy calculation for a helium ion matches that of a hydrogen atom, hinting at a link between energy differences. Johannes Rydberg's 1888 formula for "hydrogen-like" atoms, based on spectral line patterns, influenced Niels Bohr's quantization explanation in 1913.
Emission spectra
how emission spectra are produced when energy is supplied to a gas of atoms at low pressure, causing them to emit electromagnetic radiation. This process involves using an electrical discharge to excite the atoms, dispersing the emitted radiation using a spectrometer, and observing the resulting discrete lines in different regions of the electromagnetic spectrum based on the energy levels of the electrons.
Absorption spectra
Absorption spectra show bands of colors in emitted light
Solids have continuous spectrum due to closely packed atoms
Energy levels combine to form energy bands in solids
Absorption occurs when electrons absorb photons of specific frequencies
Absorbed frequencies are emitted in random directions
Absorption spectra for sodium can be demonstrated using a spectrometer and sodium chloride
Nuclei with specific protons and neutrons are nuclides.
About 60 of these nuclides are radioactive.
Radioactive decay involves parent nuclide changing to daughter nuclide(s).
Nucleus contains protons and neutrons, collectively called nucleons.
Strong nuclear force binds nucleons, overcoming electrostatic repulsion.
Neutrons moderate repulsion, making nuclei stable.
Heavier nuclei require more neutrons for stability.
Explanation of Fraunhofer Lines
Discovery: William Woolaston observed absorption lines in the Sun's spectrum in 1802.
Invention: Joseph von Fraunhofer built a spectrometer in 1814 and invented the diffraction grating to analyze these lines.
Significance: These lines, known as Fraunhofer lines, were the first spectral lines observed, labeled A-K.
Purpose: Astronomers use these lines to study the composition of stars, providing valuable insights into stellar properties.
Nuclide Nomenclature
Description: Shorthand method for nuclide composition.
Element Representation: Chemical symbol (H, He, Li, Be, B, etc.).
Nucleus Structure: Proton number (Z) and nucleon number (A).
Format: ZAX (e.g., 6 12C for carbon-12, 6 14C for carbon-14).
Isotopes: Nuclides of the same element with different neutron numbers.
Half-life and Radioactive Decay
Random Process: Radioactive decay is random, but statistically predictable with a large sample.
Constant Probability: Nuclides have a constant probability of decay independent of sample size.
Half-life: Time for half the nuclei in a sample to decay, varies among nuclides.
Decay Independence: Decay is independent of physical state and conditions except for nuclear interactions.
Exponential Decay: Common to all radioactive isotopes, never reaching zero.
Modeling Decay: Can be simulated experimentally or with software/dice.
Measuring Decay: Geiger-Müller tube for beta/gamma radiation count rate measurement.
Absorption: Alpha, beta, and gamma radiation interact differently with matter.
Absorption Measurement: Thickness of materials needed to absorb radiation can be measured.
Background Radiation: Various sources contribute to background radiation exposure.
Sievert Unit: Measures radiation effects, not examined in IB Diploma Programme physics course.