L02 Atomic bonding
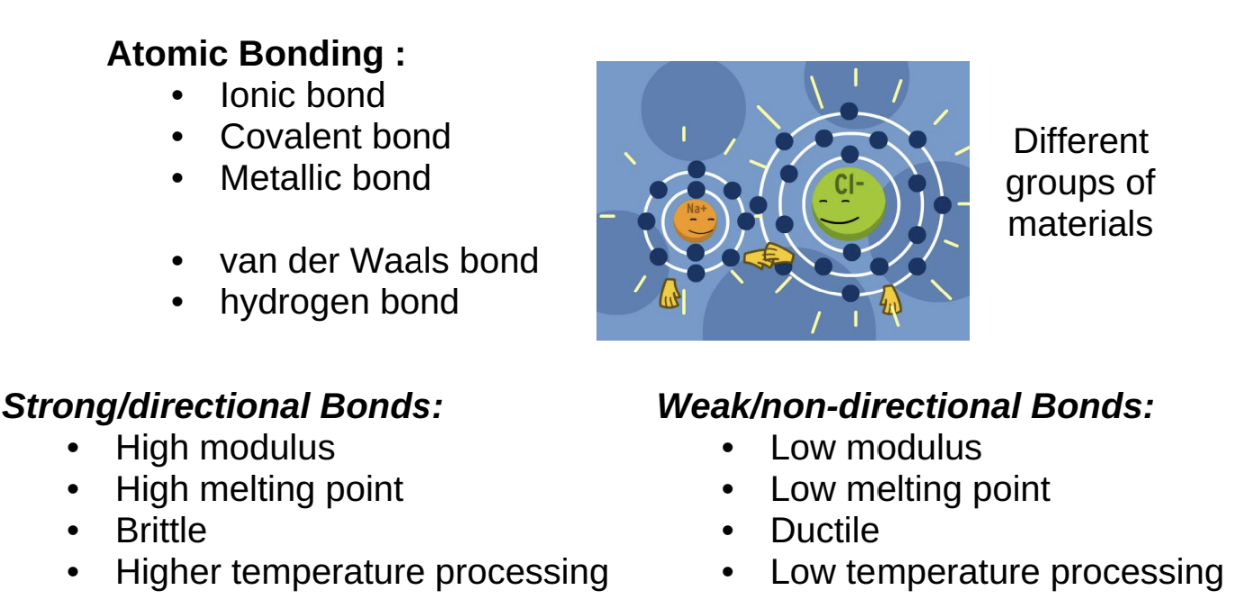
Types of Atomic Bonding
Valence Electrons:
Outermost shell electrons involved in bonding between atoms
Achieve stable configurations (outermost shell is full) by:
Giving electrons when shell is < half-full.
Receiving electrons when shell is > half-full.
Sharing electrons when shell is ~ half-full.
Primary Bonds:
3 types that form between pairs of atoms
Ionic, Covalent, and Metallic bonds
Secondary Bonds:
Weaker than primary so easier to break.
Strong Atomic Bonds
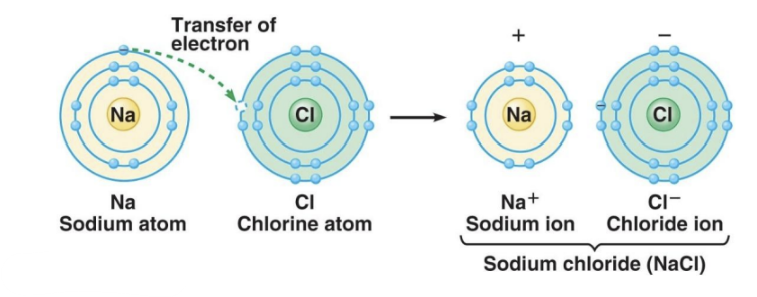
Ionic Bonding:
ionic compounds or salts
Formed between metal and non-metal ions (e.g., NaCl).
Involves the exchange of electrons between atoms
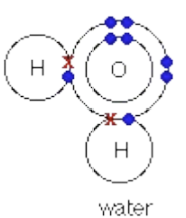
Covalent Bonding:
Found in molecules and covalent network solids (Non-metals ions: CH4, H2O).
sharing of electrons among atoms
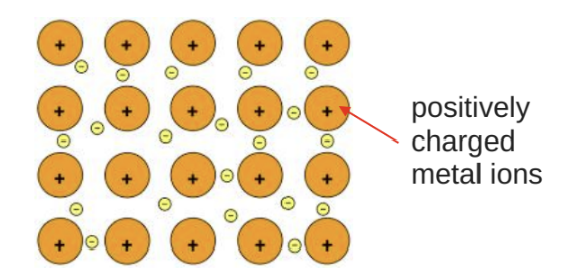
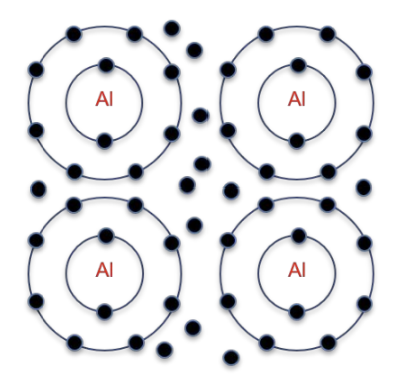
Metallic Bonding:
Present in metals and alloys.
Described as a 'sea' of electrons around positively charged metal ions.
Ionic Bonding
Definition: Formed between cations (positive ions) and anions (negative ions).
cations = atoms that have lost valence electrons to become positively charged ions e.g. Al3+
anions = atoms that have gained valence electrons to become negatively charged ions e.g. O2-
Charge neutrality is maintained:
Example: 2Al3+ + 3O2- → Al2O3.#
Ionically bonded materials:
bonds = strong and dispersed
strength of bond depends on difference in electronegativity between two elements
strength of bond increases with charge on ion.
Covalent Bonding
Definition: Bonds between non-metal atoms, common in ceramics and polymers.
Electron Sharing:
Electrons shared between two bonded atoms often leading to unequal sharing and partial ionic character.
this occurs unless 2 elements on either side of bond = identical, this will form a diamond
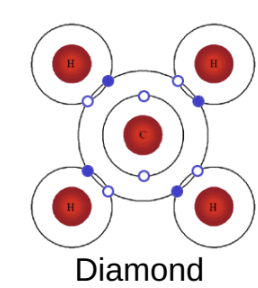
bonds in covalently bonded materials:
very strong
localised
mutually repelling
Understanding Metallic Bonding
Occurrence: mostly metals.
Properties: Typically have 1-3 (small number) of electrons on the outermost shell
Model: Valence electrons form a 'sea' of electrons
Weak Atomic Bonds
Types: Van der Waals bonds and Hydrogen bonds.
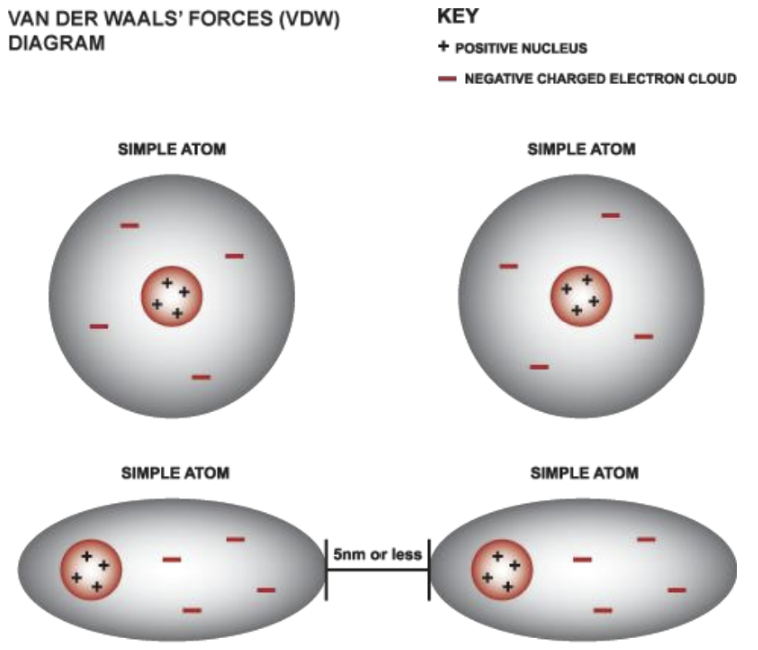
Van der Waals force: interaction or repulsion energy between molecules
Hydrogen bonds: between hydrogen atoms
Hydrogen bonds and van der Waals force normally found in gas molecules, polymers
Characteristics:
Weaker than covalent bonds; can become inactive at higher temperatures.
Mechanism of Weak Bonds
Formation: Random fluctuations cause assymetry in electron distribution, creating dipoles. this creates dipoles in neighbouring atoms
Influence: Positive end of one dipole attracts the negative end of another = Van der Waals bonding.
interaction occurs when atoms are within 5nm of each other, creating slight polarity & attraction
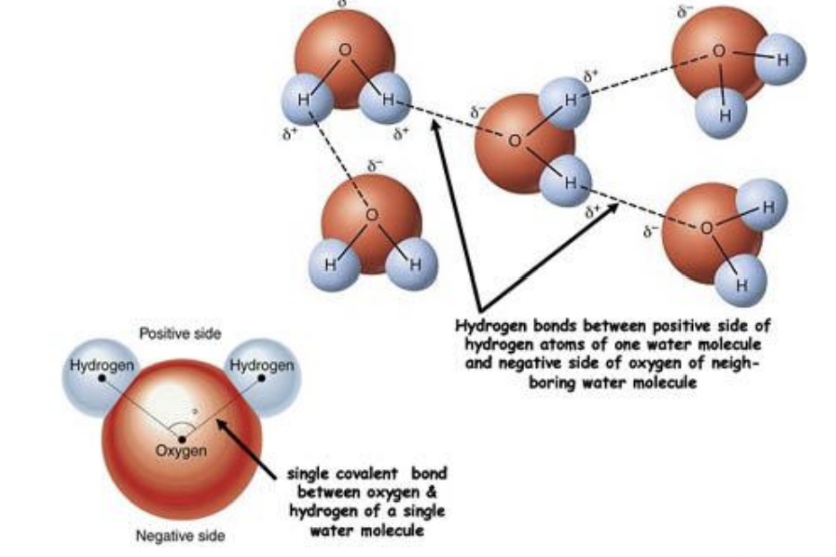
Hydrogen Bonds
When hydrogen bonds to another atom, it shares its single valence electron, leaving behind a positive charge on hydrogen atom, which is unscreened as theres no other electrons
This permanent dipole form bonds with other atoms' negative ends.
Crystalline vs. Amorphous Materials
Crystalline Materials: Long-range ordered atomic structure.
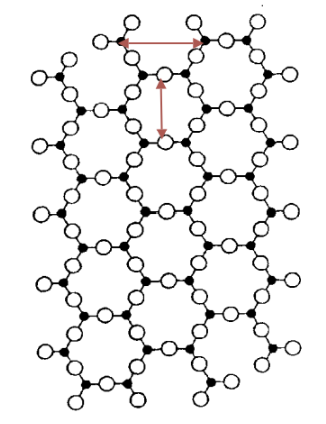
Types of Materials:
Metals and ceramics can be fully crystalline.
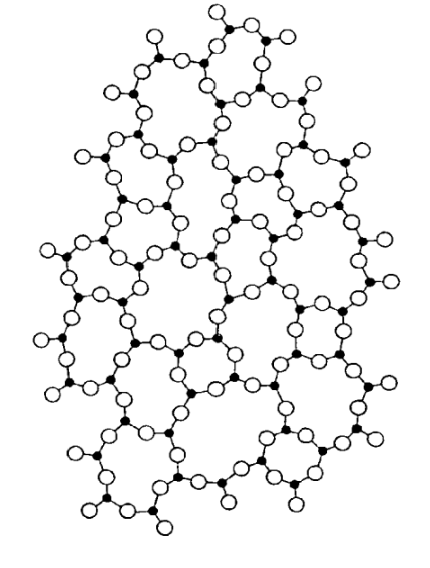
Polymers = never entirely crystalline; they are amorphous or glassy.
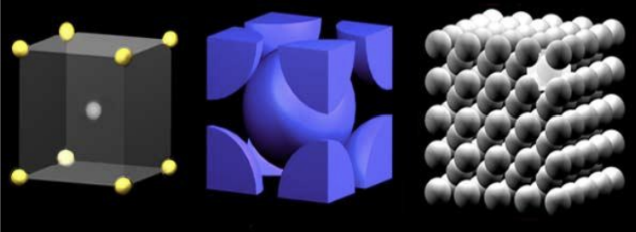
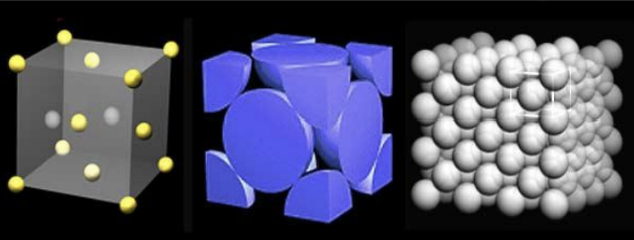
3 Principle Crystal Structures for Metals
Types of Structures:
Body-centered cubic (BCC).
Face-centered cubic (FCC).
Hexagonal close packed (HCP).
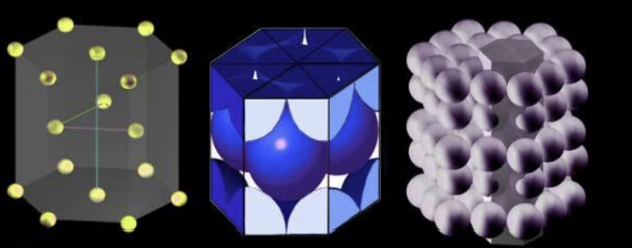
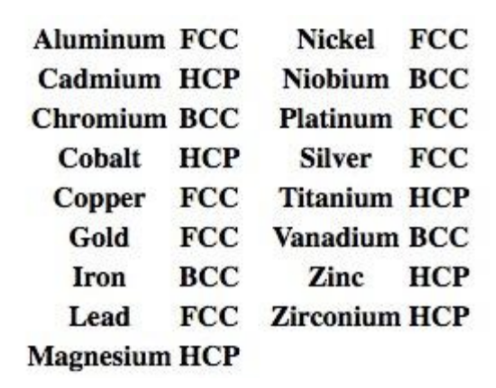
Limits of Theoretical Properties
Observation: Material properties often differ from theoretical predictions due to:
Variability in atom-atom interactions.
Imperfections in materials.
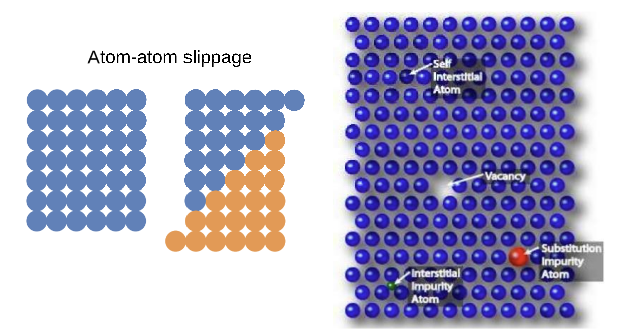
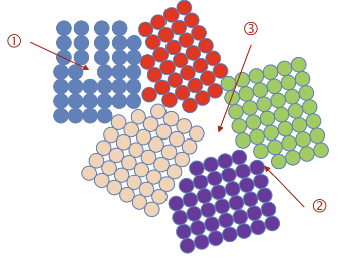
Types of Defects in Metals
Defects include:
Defects in atomic stacking.
Grains and grain boundaries.
Missing grains (pores).
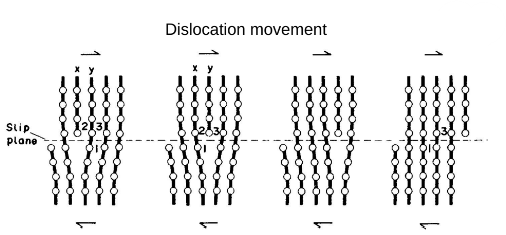
Dislocation Movement: Affects the physical properties of metals.
Characteristics of Ceramics
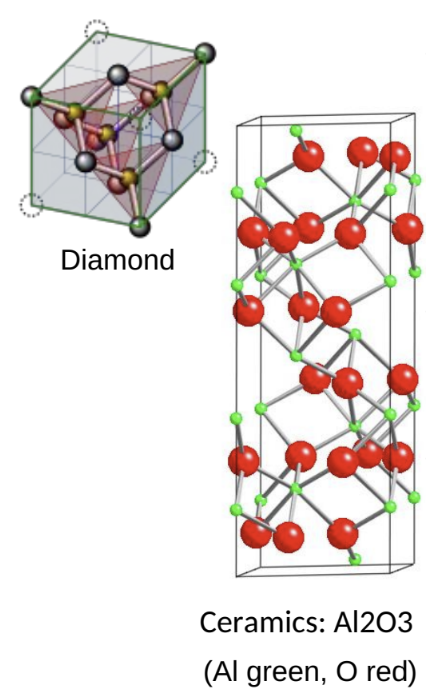
ceramics Predominantly covalently or ionically bonded materials. With few exception (e.g. diamond)
ceramics are compounds made of 2 or more atom types (e.g., Al2O3).
covalent materials = bonds highly directional and mutually repelling, leading to open structures
ionically bonded = close packed, with as many anions surrounding a central cation and viceversa to try to maximise electrostatic attraction and minimise repulsion.
Properties of Polymers
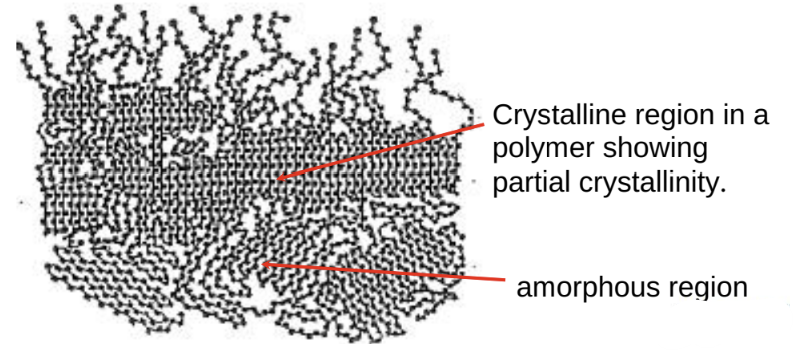
Structure: long chains, irregularly packed. amorphous
Crystallinity: regions within some polymers where the carbon-carbon chains are packed together in a regular, repeating pattern
this depends on the atoms bonded to the backbone.
polymers Can exist as wholly amorphous or partially crystalline.
Summary of Atomic Bonding