Physiology - Week 5 Study Guide: Structure and Function of Blood Vessels and Hemodynamic Disorders
Describe the structure and function of normal blood vessels, including arteries, veins, capillaries, and the relationship to the distribution of blood volume and blood pressure throughout the circulatory system.
Overview of Blood Vessels
Arteries:
Thick walls, high pressure
Types:
Large (elastic) arteries (e.g., aorta, common carotid): lots of elastic fibers, pulsatile flow.
Medium (muscular) arteries (e.g., coronary, renal): mostly smooth muscle cells.
Small arteries/arterioles: primarily smooth muscle cells, regulate blood pressure.
Veins:
Large lumen, low pressure, compressible walls, valves present.
Function: Return blood to the heart under low pressure.
Capillaries:
Very thin walls, large numbers, slow blood flow.
Role: Exchange of oxygen and nutrients with tissues.
Lymphatic System:
Drains excess interstitial fluid, passes through lymph nodes to check for infection.
Overview of Arteries
Large (Elastic) Arteries:
Examples: Aorta, common carotid, iliac arteries.
Contain many elastic fibers, allowing them to stretch and recoil with the heartbeat.
Function: Accommodate high pressure from the heart and maintain continuous blood flow.
Medium (Muscular) Arteries:
Examples: Coronary and renal arteries.
Primarily made of smooth muscle, controlling the diameter and blood flow to organs.
Function: Distribute blood to specific organs.
Small Arteries/Arterioles:
Composed mostly of smooth muscle cells.
Function: Major site of resistance and regulation of blood pressure through vasoconstriction and vasodilation.
Analyze the elasticity of arterioles as well as the function and control of vasoconstriction and vasodilation on blood pressure and the flow of blood.
Elasticity of Arterioles
Regulation of Vascular Tone:
The elasticity of arterioles helps them adjust their diameter through vasoconstriction and vasodilation.
These changes in tone are influenced by:
Autonomic nervous system signals (e.g., sympathetic stimulation causes vasoconstriction).
Hormonal control (e.g., adrenaline and angiotensin cause vasoconstriction, while nitric oxide causes vasodilation).
Impact on Blood Pressure:
Vasoconstriction: The smooth muscle contracts, reducing the diameter of the arteriole, increasing resistance to blood flow, and raising blood pressure.
Vasodilation: The smooth muscle relaxes, increasing the diameter of the arteriole, reducing resistance, and lowering blood pressure.
Loss of Elasticity:
Conditions like atherosclerosis or hypertension can reduce the elasticity of arterioles.
This leads to increased vascular resistance and higher blood pressure, contributing to the development of cardiovascular diseases.
Overview of Veins, Capillaries and Lymphatics
Veins:
Have larger lumens and thinner walls compared to arteries.
Operate under low pressure and have valves to prevent backflow of blood.
Function: Return blood to the heart from the body.
Capillaries:
The smallest blood vessels, approximately the size of a red blood cell in diameter.
Thin-walled and numerous, allowing for the slow exchange of gases, nutrients, and waste.
Function: Facilitate the exchange between blood and tissues.
Lymphatic System:
A network of vessels that collect excess interstitial fluid and return it to the bloodstream.
Function: Maintain fluid balance and immune function by filtering pathogens.
Measurement of Blood Pressure
Systolic Pressure:
The pressure in the arteries when the heart contracts (beats).
Typically the higher number in a blood pressure reading (e.g., 120 in 120/80).
Diastolic Pressure:
The pressure in the arteries when the heart is at rest between beats.
Typically the lower number in a blood pressure reading (e.g., 80 in 120/80).
Korotkoff Sounds:
Sounds heard through a stethoscope while measuring blood pressure using a cuff.
First Korotkoff sound: indicates systolic pressure.
Last Korotkoff sound: indicates diastolic pressure.
Proper Measurement Technique:
Place the cuff on the upper arm and inflate to above systolic pressure.
Slowly deflate while listening for Korotkoff sounds.
Record both the systolic and diastolic pressure accurately.
The Role of Arteries in Blood Pressure Regulation
Elastic Arteries:
Act as pressure reservoirs.
Stretch when blood is ejected from the heart and recoil to maintain blood pressure during heart relaxation (diastole).
Function: Help smooth out the pulsatile nature of blood flow from the heart, maintaining continuous flow.
Muscular Arteries:
Distribute blood to specific organs and regions of the body.
The smooth muscle in their walls can contract or relax to regulate blood flow.
Function: Control blood pressure by adjusting their diameter through vasoconstriction and vasodilation.
Arterioles:
The primary site of resistance in the circulatory system.
Regulate blood flow into capillaries through vasoconstriction (narrowing) and vasodilation (widening).
Function: Control systemic blood pressure by adjusting resistance.
Blood Pressure Control:
Vasoconstriction: Increases resistance, raising blood pressure.
Vasodilation: Decreases resistance, lowering blood pressure.
Arterioles respond to neural signals (from the autonomic nervous system) and hormones (e.g., adrenaline) to regulate blood pressure.
Function of Veins in Blood Volume Regulation
Veins as Blood Reservoirs:
Veins hold about 60-70% of the total blood volume at any given time.
Due to their large lumen and thin walls, veins can stretch to accommodate changes in blood volume without a significant increase in pressure.
Low-Pressure System:
Veins operate under lower pressure than arteries.
Blood returns to the heart via veins through the assistance of valves and skeletal muscle contractions (the muscle pump).
Venous Valves:
Prevent backflow of blood, ensuring it moves in one direction towards the heart.
Important in lower extremities where blood must move against gravity.
Role in Circulation:
Veins adjust to changes in blood volume, helping to regulate cardiac output and blood pressure.
When more blood is needed, veins constrict (venoconstriction) to push blood towards the heart, increasing venous return.
Factors Affecting Venous Return:
Gravity, breathing (thoracic pressure changes), and body position all impact how efficiently veins return blood to the heart.
Structure and Function of Lymphatics
Lymphatic System Overview:
A network of vessels, nodes, and organs that collect excess interstitial fluid and return it to the bloodstream.
Plays a key role in maintaining fluid balance and supporting the immune system.
Function of Lymphatics:
Fluid Return: Collect and return excess interstitial fluid to the bloodstream, preventing edema.
Immune Surveillance: Lymph passes through lymph nodes, where immune cells monitor for infections or abnormalities.
Fat Absorption: Specialized lymphatic vessels called lacteals absorb fats from the digestive system and transport them to the bloodstream.
Lymphatic Vessels:
Similar to veins but thinner walls and more valves.
Begin as blind-ended capillaries in tissues, collecting excess fluid that leaks from blood capillaries.
Lymph Nodes:
Small, bean-shaped structures along lymphatic vessels.
Function: Filter lymph fluid, trapping pathogens, debris, and cancer cells.
Contain immune cells (e.g., lymphocytes) that initiate immune responses when necessary.
Importance in Circulation:
By returning fluid to the bloodstream, the lymphatic system helps maintain blood volume and pressure.
Vessel Structure and Function
Endothelial Cells (ECs): Line blood vessels, controlling blood flow, clotting, and inflammation.
Smooth Muscle Cells (SMCs): Regulate contraction and blood pressure.
Balance: Proper interaction between ECs and SMCs ensures healthy vessel function.
Causes of Vessel Damage
Hypertension: High blood pressure stresses vessel walls, damaging ECs.
Atherosclerosis: Plaque buildup (from fats and cholesterol) narrows arteries.
Inflammation: Diseases like vasculitis cause vessel inflammation and damage.
Toxins: Smoking and other toxic exposures damage vessel linings, accelerating plaque formation.
Physical Injury: Trauma or infections can also harm vessels.
Vessel Response to Injury
Blood Vessel Injury: Trauma, infection, or inflammation disrupts blood flow, often leading to ARTERIOsclerosis (general arterial stiffening).
Arteriosclerosis is a general term that refers to the thickening, hardening, and loss of elasticity in the walls of the arteries
This stiffening of the arteries can reduce blood flow to organs and tissues, increasing the risk of hypertension and other cardiovascular issues
Affects the small arteries and arterioles and is usually associated with aging
Endothelial Cell Loss: Losing ECs triggers repair processes, activating SMCs and the immune system, which can contribute to ATHEROsclerosis (plaque buildup).
Atherosclerosis is a specific type of arteriosclerosis that involves the buildup of plaques (made of fat, cholesterol, and other substances) inside the artery walls
These plaques can narrow the arteries, restrict blood flow, and lead to complications such as heart attacks, strokes, or peripheral artery disease
Commonly affects larger arteries like those in the heart, brain, and legs.
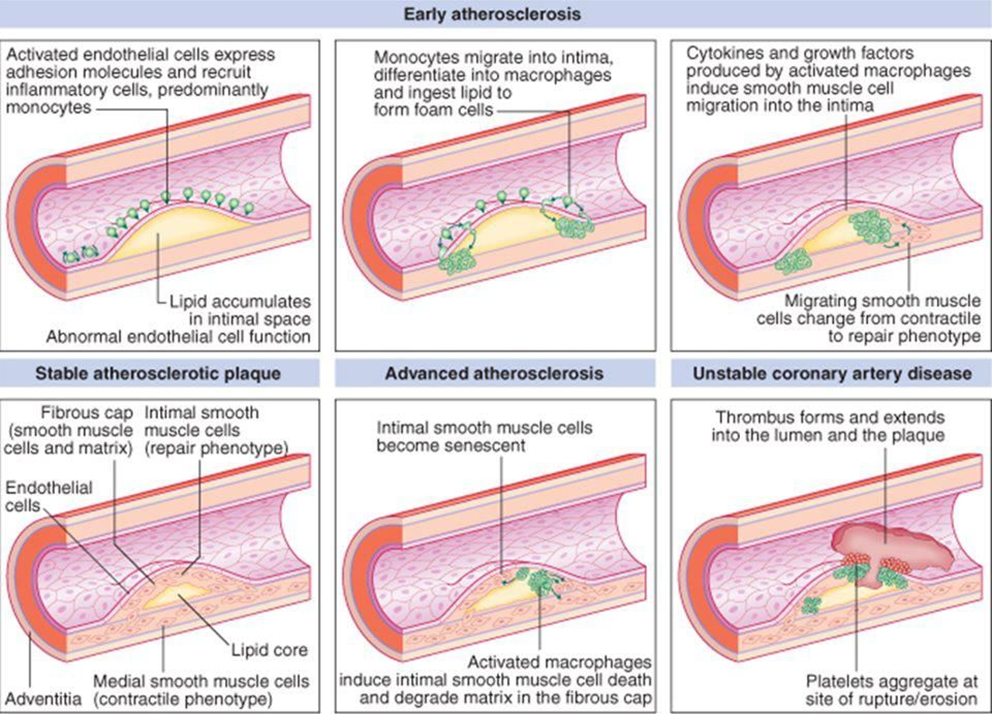
SMC Activation and Intimal Thickening: SMCs migrate to the vessel's inner layer (intima), multiply, and cause thickening—common in atherosclerosis. This can result in stenosis (narrowing), reducing blood flow.
SUMMARY = while ARTERIOsclerosis refers to the general hardening of the arteries, ATHEROsclerosis specifically refers to the plaque buildup that can lead to more serious cardiovascular events.
Consequences of Vessel Damage
Intimal Thickening: SMCs thicken vessel walls, leading to stenosis (narrowing).
Ischemia: Reduced blood flow leads to oxygen deprivation in tissues.
Clinical Effects:
Heart: Stenosis can cause angina or myocardial infarction.
Brain: Narrowed carotid arteries increase stroke risk.
Limbs: Reduced flow can result in gangrene or severe pain.
ATHEROsclerosis
Atherosclerosis Overview:
A chronic condition characterized by the buildup of plaque (lipids, cholesterol, calcium, and other substances) in the inner walls of arteries.
Plaques narrow the arteries, reducing blood flow and increasing the risk of blockages.
Development of Atherosclerosis:
Begins with endothelial injury, which leads to inflammation and the accumulation of lipids.
Over time, plaques grow larger, causing luminal stenosis.
Rupture of plaques can lead to the formation of blood clots (thrombosis), which can obstruct the artery entirely.
Risk Factors:
High cholesterol, smoking, hypertension, diabetes, and a sedentary lifestyle increase the risk of atherosclerosis.
Family history and aging are also significant factors.
Modifiable vs. nonmodifiable risk factors
Clinical Consequences:
Coronary artery disease: Atherosclerosis in the coronary arteries can cause angina or myocardial infarction (heart attack).
Cerebrovascular disease: Plaque buildup in the carotid arteries can lead to stroke.
Peripheral artery disease: Affects blood flow to the extremities, causing pain and tissue damage.
ATHEROsclerosis Modifiable Risk Factors
Hyperlipidemia:
Elevated levels of LDL cholesterol are a major risk factor for atherosclerosis.
HDL cholesterol helps remove cholesterol from plaques, reducing risk.
Hypertension:
High blood pressure damages the endothelium, accelerating plaque formation.
Smoking:
Damages the endothelium and increases oxidative stress and inflammation, promoting plaque development.
Diabetes:
High blood sugar levels lead to endothelial dysfunction and increased lipid deposition in arteries.
Obesity and Physical Inactivity:
Both are linked to metabolic disturbances that increase the risk of developing atherosclerosis.
ATHEROsclerosis NON-Modifiable Risk Factors
Age:
The risk of atherosclerosis increases with age.
Gender:
Men are at higher risk than premenopausal women, though the risk increases in women after menopause.
Family History:
A genetic predisposition to cardiovascular disease increases the risk of developing atherosclerosis.
ATHEROsclerosis Additional Risk Factors
C-reactive protein (CRP):
Elevated CRP levels indicate systemic inflammation, which is linked to increased atherosclerosis risk.
Sedentary Lifestyle:
Lack of regular physical activity contributes to several modifiable risk factors, including obesity and hypertension.
Other Common Vascular Diseases: Dissections
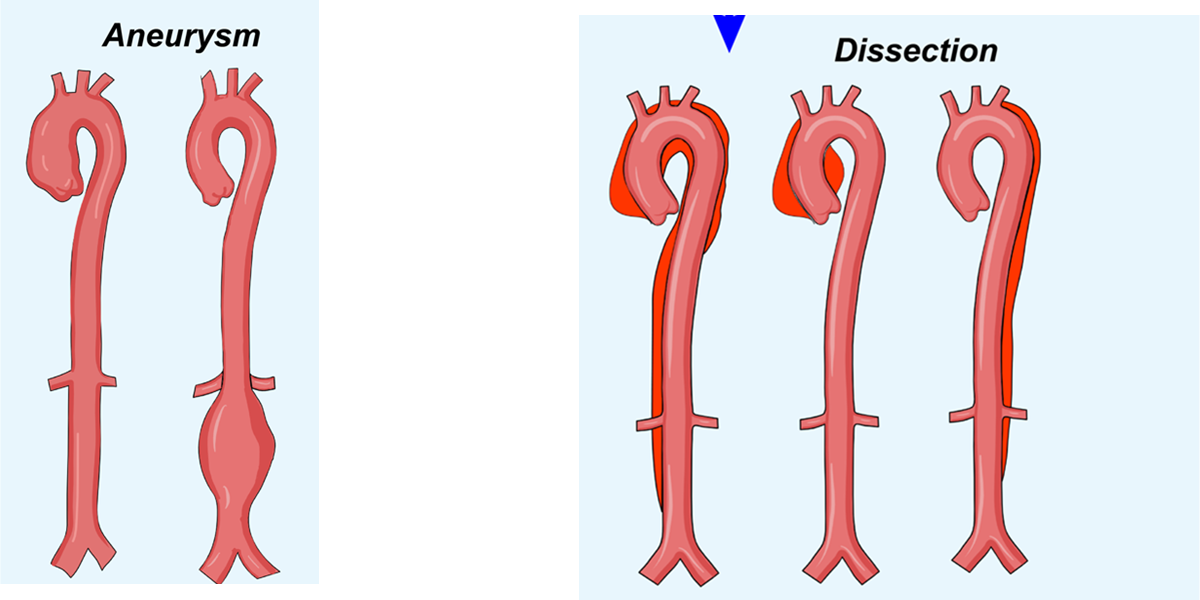
Aortic Dissection:
Occurs when blood enters the media layer of the aortic wall, separating the layers and creating a false lumen.
Type A Dissection
Involves the ascending aorta and is more dangerous; requires immediate surgery.
Type B Dissection
Involves the descending aorta and may be managed with medication if stable.
Risk Factors for Aneurysms and Dissections:
Hypertension: Increases the risk of both aneurysms and dissections.
Connective tissue disorders (e.g., Marfan syndrome) also predispose individuals to these conditions.
Atherosclerosis: Aneurysms are often associated with underlying atherosclerosis
Other Common Vascular Diseases: Aneurysms
Aneurysms:
A localized dilation or bulging of a blood vessel wall, usually due to a weakness in the vessel wall.
Commonly occurs in the aorta (abdominal aortic aneurysm) or cerebral arteries.
Types:
True Aneurysm:
Involves all three layers of the arterial wall (e.g., fusiform or saccular).
False Aneurysm:
A breach in the vessel wall leads to a collection of blood outside the vessel, contained by surrounding tissue.
Clinical Consequences of Aneurysms:
Rupture
Can cause life-threatening hemorrhage. For example, a ruptured aortic aneurysm can lead to sudden death.
Compression of nearby structures
Large aneurysms may compress organs or nerves.
Thrombus formation
Blood clots may form in the dilated area, leading to embolization.
Aneurysm vs Dissection
Aneurysm
A localized bulge in the vessel due to wall weakening.
Dissection
A tear in the inner layer of the artery, leading to separation between the vessel layers
Both conditions are dangerous, but their underlying mechanisms differ—aneurysms involve dilation, while dissections involve a tear and separation within the vessel wall.
Describe the distribution of fluid between the intracellular and extracellular compartments and identify the basic aspects of normal circulation.
Intracellular Fluid (ICF):
Location: Inside cells, makes up two-thirds of body fluid.
Major Ions: Potassium (K⁺) and Phosphate (PO₄³⁻).
Functions:
Maintains cell shape, supports metabolism.
Regulates osmotic balance and nutrient/waste exchange.
Extracellular Fluid (ECF):
Location: Outside cells, one-third of body fluid.
Components:
Interstitial Fluid (around cells).
Plasma (blood component).
Major Ions: Sodium (Na⁺) and Chloride (Cl⁻).
Functions:
Transports nutrients, oxygen, and waste.
Regulates blood pressure and osmotic pressure.
Capillary Exchange Mechanisms (Review)
Capillary Exchange Mechanisms:
Forces Driving Fluid Movement:
Hydrostatic Pressure: Pushes fluid out of capillaries.
Osmotic (Oncotic) Pressure: Pulls fluid into capillaries via plasma proteins (albumin).
Balance:
Filtration at arterial end (hydrostatic > osmotic).
Reabsorption at venous end (osmotic > hydrostatic).
Lymphatic System: Drains excess fluid, preventing edema
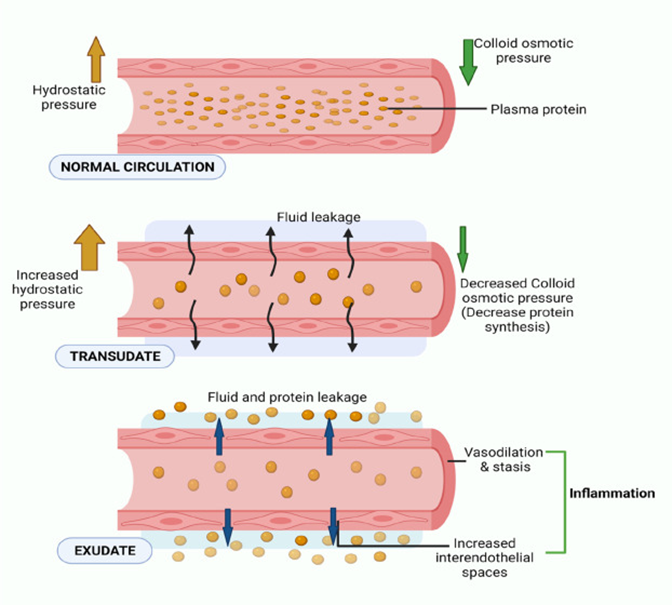
Edema: Causes and Types (Transudate vs. Exudate)
Definition of Edema:
Edema is the accumulation of excess fluid in the interstitial tissue spaces, leading to swelling.
Causes of Edema:
Increased Hydrostatic Pressure:
Often due to impaired venous return (e.g., heart failure, deep vein thrombosis), causing fluid to be pushed out of capillaries into the interstitial space.
Decreased Plasma Oncotic Pressure:
Caused by a reduction in plasma proteins (e.g., hypoalbuminemia from liver disease or nephrotic syndrome), leading to less fluid being reabsorbed into capillaries.
Lymphatic Obstruction:
Impaired lymphatic drainage (e.g., from cancer, surgery, or infections) results in fluid accumulation in tissues.
Increased Vascular Permeability:
Inflammatory conditions (e.g., infections, allergic reactions) increase the permeability of capillaries, allowing fluid and proteins to leak into the interstitial space.
Sodium and Water Retention:
Disorders such as renal failure can lead to excessive sodium retention, increasing blood volume and pressure, promoting edema.
Transudate:
Protein-poor fluid caused by imbalances in hydrostatic or oncotic pressure.
Typically associated with non-inflammatory conditions like heart failure or liver disease.
Fluid is clear and low in cells.
Exudate:
Protein-rich fluid caused by increased capillary permeability, often in response to inflammation.
Associated with conditions such as infections, trauma, or malignancy.
Fluid is cloudy and contains immune cells, proteins, and other debris.
Pathophysiology of Hyperemia
Hyperemia Overview:
Hyperemia refers to an increase in blood flow to a particular tissue, resulting in redness and warmth in the affected area.
Types of Hyperemia:
Active Hyperemia:
Caused by arteriolar dilation due to increased tissue demand for oxygen (e.g., during exercise or inflammation).
Blood vessels dilate to increase the flow of oxygenated blood to the tissues.
Passive Hyperemia (Congestion):
Caused by impaired venous outflow, leading to an accumulation of deoxygenated blood in the tissues (e.g., in heart failure or venous obstruction).
Affected tissues appear blue or cyanotic due to low oxygen levels.
Clinical Relevance:
Acute hyperemia can occur in response to injury or inflammation, while chronic congestion (e.g., in liver cirrhosis or chronic heart failure) can lead to tissue damage and fibrosis over time.
Role of Lymphatic Drainage in Fluid Regulation
Lymphatic System Overview:
A network of vessels that collects excess interstitial fluid and returns it to the bloodstream.
Acts as a safety valve to prevent the accumulation of fluid in tissues.
Lymphatic Capillaries:
Blind-ended, thin-walled structures that are highly permeable to proteins and larger molecules that cannot reenter capillaries.
Drain excess fluid from the interstitial space, transporting it through lymph nodes for immune surveillance.
Prevention of Edema:
The lymphatic system helps maintain fluid balance by removing any excess fluid that remains after capillary filtration and reabsorption.
If lymphatic drainage is impaired (e.g., due to blockage or removal of lymph nodes), lymphedema can develop.
Immune Function:
Lymph nodes along the lymphatic vessels filter fluid, trapping pathogens, and debris.
Plays a crucial role in initiating immune responses by activating lymphocytes when pathogens are detected.
Return to Circulation:
The lymphatic fluid, now called lymph, is eventually returned to the bloodstream via the thoracic duct, maintaining blood volume and pressure.
Explain normal hemostasis and the role of endothelial cells, platelets, and the coagulation proteins in the extrinsic and intrinsic clotting cascade along with associated dysfunction.
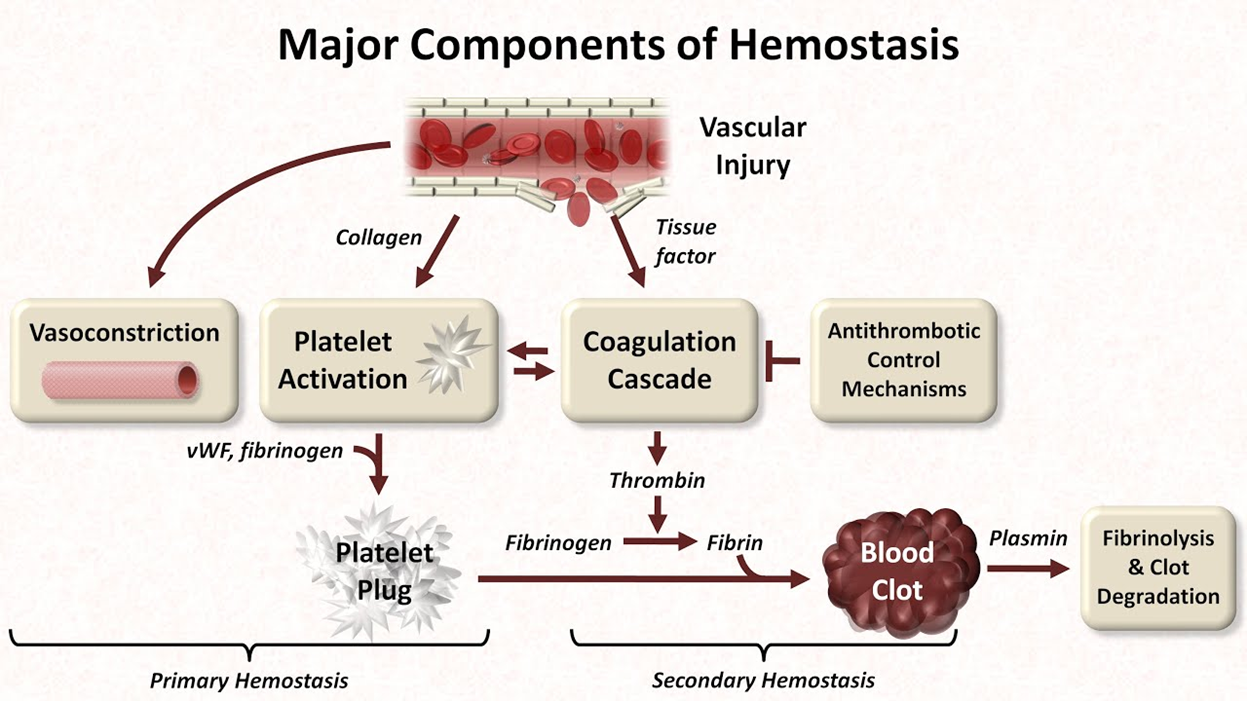
Normal Hemostasis Process: Vasoconstriction and Platelet Plug Formation
Hemostasis Overview:
Hemostasis is the body’s natural process of stopping bleeding from an injured vessel, ensuring proper clot formation while maintaining blood flow.
Step 1: Vasoconstriction:
Vascular spasm occurs immediately after vessel injury to reduce blood flow and limit blood loss.
Triggered by local nervous reflexes and the release of endothelin, a vasoconstrictor released by endothelial cells.
Temporary, but crucial in allowing time for other hemostatic processes to activate.
Step 2: Platelet Plug Formation (Primary Hemostasis):
Platelets adhere to the exposed collagen in the damaged vessel wall, facilitated by von Willebrand factor (vWF).
Platelets become activated and change shape, releasing chemicals like ADP and Thromboxane A2, which attract more platelets to the site.
Platelets aggregate to form a primary hemostatic plug, temporarily sealing the injury.
Platelet Activation:
Platelets release granules containing clotting factors that further enhance aggregation and strengthen the platelet plug.
This plug is fragile and needs to be stabilized by the coagulation process
Normal Hemostasis Process: Coagulation (Secondary Homeostasis)
Step 3: Coagulation Cascade:
Secondary hemostasis stabilizes the platelet plug through the formation of a fibrin clot.
Involves a series of enzymatic reactions that activate clotting factors (e.g., Factors VII, X, and thrombin).
The cascade is divided into two pathways:
Intrinsic Pathway: Activated by trauma inside the blood vessel, involving Factors XII, XI, IX, and VIII.
Extrinsic Pathway: Activated by external trauma, involving Tissue Factor (TF) and Factor VII.
Both pathways converge at the common pathway, where Factor X is activated, leading to the conversion of prothrombin to thrombin.
Formation of Fibrin Mesh:
Thrombin converts fibrinogen (soluble) into fibrin (insoluble), which forms a mesh that strengthens and stabilizes the platelet plug.
The result is a durable secondary hemostatic plug that effectively stops bleeding.
Clot Retraction and Dissolution:
After the clot forms, platelets contract, pulling the edges of the wound closer together (clot retraction).
Eventually, the clot is dissolved by plasmin in a process called fibrinolysis, restoring normal blood flow after healing.
Role of Endothelial Cells in Hemostasis
Endothelial Cells Overview:
Endothelial cells (ECs) line the interior surface of blood vessels and play a key role in maintaining vascular integrity.
ECs act as a barrier between the blood and the vessel wall, regulating blood flow and preventing abnormal clot formation.
Antithrombotic Properties of Endothelial Cells:
Antiplatelet Effects:
Intact ECs produce prostacyclin (PGI2) and nitric oxide (NO), which inhibit platelet aggregation and promote vasodilation, preventing unnecessary clotting.
ECs also release adenosine diphosphatase, which degrades ADP to inhibit platelet adhesion.
Anticoagulant Properties:
ECs express heparin-like molecules that enhance the activity of antithrombin, a natural anticoagulant that inactivates thrombin and other clotting factors.
ECs also produce thrombomodulin, which binds thrombin and activates protein C, a key anticoagulant that degrades Factors Va and VIIIa.
Fibrinolytic Properties:
ECs produce tissue plasminogen activator (t-PA), which converts plasminogen to plasmin, dissolving clots after they have formed and ensuring blood flow is restored.
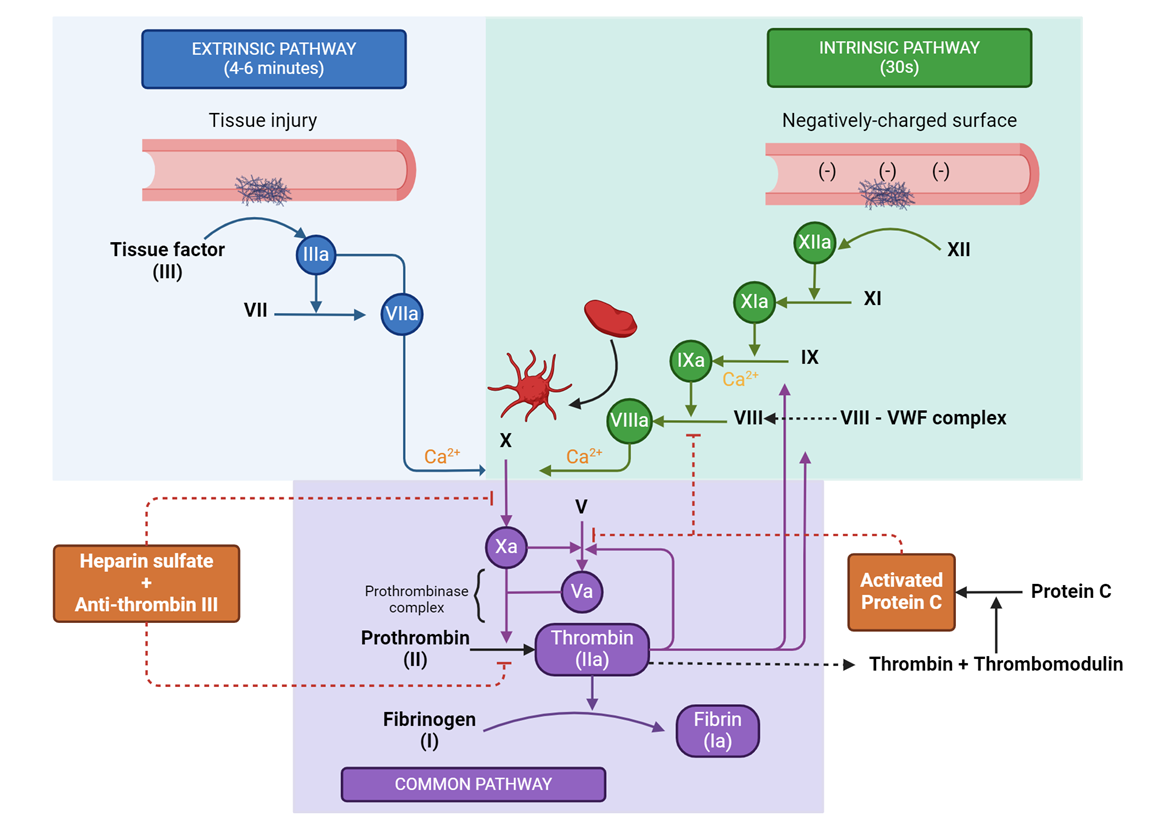
The Coagulation Cascade: Overview
Coagulation Cascade Overview:
The coagulation cascade is a series of enzymatic reactions that lead to the formation of a fibrin clot, stabilizing the platelet plug and stopping bleeding.
It involves two main pathways:
Intrinsic Pathway: Activated by damage inside the blood vessel.
Extrinsic Pathway: Activated by external trauma to the vessel.
Both pathways converge at the common pathway, leading to the production of thrombin and fibrin.
Key Players:
Clotting Factors: These are proteins (e.g., Factor I, II, V, VII, VIII, IX, X) that are activated sequentially in a cascade.
Calcium and phospholipids are essential cofactors in the activation of clotting factors.
The Intrinsic Pathway
Activation of Intrinsic Pathway:
Triggered by damage to the blood vessel itself (e.g., exposure of collagen or abnormal blood flow).
Involves a series of clotting factors (XII, XI, IX, and VIII) that are activated in sequence.
Key Steps:
Factor XII is activated upon contact with exposed collagen or a negatively charged surface.
Activated Factor XII (XIIa) converts Factor XI to its active form (XIa).
Factor XIa activates Factor IX to IXa.
Factor IXa, in the presence of Factor VIIIa and calcium, activates Factor X, beginning the common pathway.
Clinical Relevance:
Disorders of the intrinsic pathway (e.g., hemophilia A and B) result from deficiencies in Factor VIII or Factor IX, leading to impaired clot formation and excessive bleeding.
The Extrinsic Pathway and Common Pathway
Activation of Extrinsic Pathway:
Initiated by tissue factor (TF), a protein expressed on the surface of cells outside the blood vessel in response to external trauma.
TF binds to Factor VII, forming a complex that activates Factor VIIa.
This complex directly activates Factor X, leading to the common pathway.
Common Pathway:
Activated Factor X (Xa) converts prothrombin (Factor II) to thrombin in the presence of Factor Va, calcium, and phospholipids.
Thrombin converts fibrinogen (Factor I) into fibrin, which forms a mesh to stabilize the platelet plug.
Thrombin also activates Factor XIII, which cross-links fibrin strands, further stabilizing the clot.
Regulation and Control:
Natural anticoagulants (e.g., antithrombin, protein C, and protein S) ensure that the coagulation cascade is localized and does not lead to excessive clotting.
Clinical Relevance:
Disorders of the extrinsic pathway (e.g., Factor VII deficiency) result in impaired clotting after trauma.
The Prothrombin Time (PT) test assesses the extrinsic and common pathways, while the Activated Partial Thromboplastin Time (aPTT) test evaluates the intrinsic pathway.
Clot Retraction
Clot Retraction Overview:
Clot retraction is the process where the fibrin mesh contracts, pulling the edges of the wound closer together.
Begins shortly after the clot forms, typically within minutes, and continues for hours.
Mechanism of Clot Retraction:
Platelets trapped within the clot contain actin and myosin, similar to muscle cells.
These platelets contract, pulling on the fibrin strands, causing the clot to shrink and compress.
This helps reduce the size of the wound and minimize blood loss.
Function:
Reduces the wound gap: Facilitates faster tissue repair and healing by bringing the edges of the injury closer together.
Strengthens the clot: Compacts the clot, making it more resistant to further disruption.
Clinical Relevance:
Impaired clot retraction can indicate platelet function disorders, such as Glanzmann thrombasthenia, where platelets fail to properly bind to fibrin and cause retraction.
Clot Dissolution: Plasminogen Activation and Fibrinolysis
Clot Dissolution (Fibrinolysis):
Once the vessel has healed, the body dissolves the clot through the process of fibrinolysis.
This prevents clots from persisting unnecessarily and obstructing blood flow.
Plasminogen Activation:
Plasminogen is an inactive enzyme that is incorporated into the clot during formation.
It is activated by tissue plasminogen activator (t-PA), which is secreted by endothelial cells, and by urokinase.
When activated, plasminogen is converted into plasmin, the enzyme responsible for breaking down fibrin.
Fibrin Breakdown:
Plasmin cleaves fibrin strands, breaking down the clot into soluble fibrin degradation products (FDPs), which are removed from circulation.
This process restores normal blood flow through the vessel.
Regulation of Fibrinolysis:
Plasminogen activator inhibitors (PAIs) prevent excessive plasmin activity, ensuring that fibrinolysis only occurs when the vessel has healed.
α2-antiplasmin is a major inhibitor of plasmin, regulating its activity to prevent unwarranted clot breakdown.
Clinical Relevance:
Impaired fibrinolysis can result in thrombosis, while excessive fibrinolysis can lead to bleeding disorders.
Therapeutically, t-PA is used as a clot-busting drug to treat conditions like myocardial infarction and stroke by promoting fibrinolysis.
Define and describe hypertension, edema, thrombosis, emboli, atherosclerosis.
Vasoconstriction and Vasodilation Mechanisms
Vasoconstriction Mechanisms
Vasoconstriction:
Definition: The narrowing of blood vessels due to the contraction of smooth muscle in the arteriole walls.
Mechanism:
Triggered by sympathetic nervous system activation.
Hormones like adrenaline, angiotensin II, and vasopressin bind to receptors on smooth muscle cells, causing them to contract.
Increases resistance to blood flow, raising blood pressure.
Function: Helps redirect blood flow to essential organs (e.g., heart and brain) during stress or low blood volume.
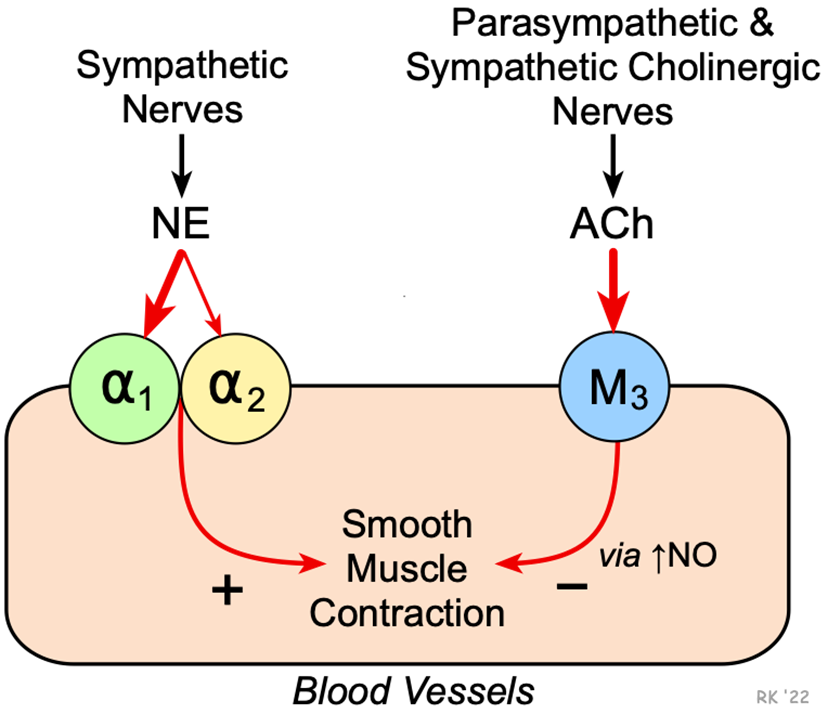
Neurotransmitters, Hormones, and Receptors in VasoCONSTRICTION
Vasoconstriction:
Neurotransmitters & Hormones:
Norepinephrine
Binds to alpha-1 adrenergic receptors, causing vessel constriction.
Epinephrine
Binds to alpha-1 receptors for constriction, and beta-2 receptors for dilation in muscles.
Angiotensin II
Activates AT1 receptors, causing potent vasoconstriction.
Vasopressin (ADH)
Binds to V1 receptors on smooth muscle, increasing constriction.
Key Receptors:
Alpha-1 Adrenergic: Norepinephrine/Epinephrine → Constriction.
AT1 Receptors: Angiotensin II → Constriction.
V1 Receptors: Vasopressin → Constriction.
VasoDILATION Mechanisms
Vasodilation:
Definition: The widening of blood vessels due to the relaxation of smooth muscle in the arteriole walls.
Mechanism:
Triggered by the parasympathetic nervous system and certain hormones (e.g., nitric oxide, bradykinin).
Nitric oxide (NO) is a major vasodilator released by endothelial cells, causing smooth muscle relaxation.
Decreases resistance to blood flow, lowering blood pressure.
Function: Increases blood flow to tissues, especially during rest, digestion, or exercise.
Neurotransmitters, Hormones, and Receptors in VasoDILATION
Vasodilation:
Neurotransmitters & Hormones:
Nitric Oxide (NO): Triggers cGMP in smooth muscle → Relaxation.
Acetylcholine (ACh): Stimulates NO release via muscarinic receptors.
Bradykinin: Activates B2 receptors to release NO.
Atrial Natriuretic Peptide (ANP): Binds to NPR receptors → cGMP → Dilation.
Key Receptors:
Beta-2 Adrenergic: Epinephrine → Dilation (muscle vessels).
M3 Muscarinic: ACh → NO release → Dilation.
B2 Receptors: Bradykinin → NO release → Dilation.
NPR Receptors: ANP → cGMP → Dilation.
Balancing Neurotransmitters and Hormones for Homeostasis
Balance & Homeostasis:
Sympathetic system → vasoconstriction (stress, blood pressure).
Parasympathetic system → vasodilation (relaxation, digestion).
Endothelium regulates balance via NO and other factors to maintain blood flow.
Understanding Systolic and Diastolic Pressure
Systolic Pressure:
The pressure in the arteries when the heart contracts and pumps blood into the aorta.
Represents the maximum pressure exerted on the arterial walls during a heartbeat.
Typically the higher number in a blood pressure reading (e.g., 120 mmHg in 120/80).
Important for assessing the force of blood flow and the workload on the heart.
Diastolic Pressure:
The pressure in the arteries when the heart is at rest between beats.
Represents the minimum pressure exerted on the arterial walls.
Typically the lower number in a blood pressure reading (e.g., 80 mmHg in 120/80).
Reflects how well the arteries maintain pressure during the heart’s relaxation phase.
Significance of Both Pressures:
Both systolic and diastolic pressure provide key information about the health of the cardiovascular system.
Elevated systolic pressure can indicate stiffening of arteries or high cardiac output, increasing the risk of heart attack or stroke.
Elevated diastolic pressure often reflects vascular resistance and may point to hypertension-related complications.
Normal vs. Abnormal Readings:
Normal Range: Systolic <120 mmHg, Diastolic <80 mmHg.
Hypertension: Systolic ≥130 mmHg or Diastolic ≥80 mmHg (depending on guidelines and age).
Clinical Relevance:
Monitoring both systolic and diastolic pressures helps detect hypertension, hypotension, and cardiovascular risk, guiding appropriate medical intervention.
Pathology of Hypertension: Overview
What is Hypertension?
Defined as persistently elevated blood pressure. Common thresholds:
Systolic ≥130 mmHg or Diastolic ≥80 mmHg.
Two main types:
Primary (Essential) Hypertension: No identifiable cause, accounting for 90-95% of cases.
Secondary Hypertension: Resulting from an identifiable cause, such as kidney disease or adrenal gland disorders.
Primary Hypertension:
Risk factors include:
Genetic predisposition.
Diet: High sodium intake.
Lifestyle factors: Lack of exercise, obesity, smoking, and stress.
Age and gender: More common with advancing age and in males up to age 65.
Secondary Hypertension:
Caused by:
Kidney disease.
Endocrine disorders (e.g., hyperaldosteronism, pheochromocytoma).
Medications: Certain drugs like NSAIDs or oral contraceptives.
Sleep apnea.
Mechanisms and Effects of Hypertension
Pathophysiology of Hypertension:
Vascular resistance: Hypertension results from increased systemic vascular resistance.
Vessel damage: Chronic high blood pressure damages arterial walls, leading to endothelial dysfunction and vessel thickening.
Imbalance in regulatory mechanisms: Dysregulation of the renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system contributes to increased vascular tone and blood volume.
End-Organ Damage:
Heart:
Hypertension increases the workload on the heart, leading to left ventricular hypertrophy (LVH) and increasing the risk of heart failure.
Coronary artery disease (CAD) is common due to damage to arterial walls.
Brain:
High blood pressure increases the risk of stroke and cerebral hemorrhage due to weakened blood vessels.
Kidneys:
Hypertension damages renal arteries, reducing kidney function and potentially leading to chronic kidney disease (CKD).
Eyes:
Damage to retinal blood vessels can lead to hypertensive retinopathy and vision loss.
A Brief Introduction to Clinical Management of Hypertension
Lifestyle Modifications:
Dietary changes: Reduce sodium intake, increase potassium, and adopt the DASH diet (Dietary Approaches to Stop Hypertension).
Physical activity: Regular exercise helps reduce blood pressure.
Weight management: Achieving and maintaining a healthy weight is key in managing hypertension.
Stress reduction: Managing stress through relaxation techniques, such as meditation, can lower blood pressure.
Pharmacological Treatment:
Diuretics: Help eliminate excess sodium and fluid from the body, lowering blood pressure.
ACE inhibitors/ARBs: Block the effects of angiotensin, reducing vasoconstriction and lowering blood pressure.
Calcium channel blockers: Relax blood vessels by preventing calcium from entering the muscle cells of the heart and arteries.
Beta-blockers: Reduce heart rate and the force of contraction, lowering blood pressure.
Monitoring and Compliance:
Regular blood pressure monitoring is crucial for managing the condition.
Adherence to medication and lifestyle modifications is essential for reducing the risk of complications.
Discuss the pathology and clinical implications behind hemophilia, thalassemia, thrombophilia, polycythemia, hemochromatosis, Raynaud phenomenon, varicose veins, vascular aneurysms, and dissections.
Pathology of Clotting Disorders: Hemophilia
A genetic disorder that results in deficient or defective clotting factors, leading to excessive bleeding.
Types:
Hemophilia A: Caused by a deficiency of Factor VIII.
Hemophilia B: Caused by a deficiency of Factor IX.
Symptoms:
Prolonged bleeding, especially after injury or surgery.
Spontaneous bleeding in joints (hemarthrosis) and muscles.
Treatment:
Replacement therapy with recombinant or plasma-derived Factor VIII or Factor IX.
In severe cases, regular prophylactic factor infusions are needed to prevent bleeding episodes.
Pathology of Clotting Disorders: Thrombophilia
A condition where the blood has an increased tendency to form clots (hypercoagulability), leading to an elevated risk of thrombosis.
Inherited Thrombophilia:
Factor V Leiden mutation: A genetic mutation that makes Factor V resistant to degradation by Protein C, increasing the risk of deep vein thrombosis (DVT).
Prothrombin gene mutation (G20210A): Increases prothrombin levels, enhancing the tendency to form clots.
Acquired Thrombophilia:
Conditions like antiphospholipid syndrome (APS), which promotes clotting through the production of antibodies that target phospholipid-binding proteins.
Prolonged immobility, cancer, pregnancy, or use of oral contraceptives can increase the risk of thrombophilia.
Symptoms:
Recurrent deep vein thrombosis (DVT), pulmonary embolism, or other thrombotic events.
Treatment:
Anticoagulants like warfarin, heparin, or direct oral anticoagulants (DOACs) are commonly used to prevent clot formation
Pathophysiology of Veins
Varicose Veins:
Caused by venous valve incompetence, leading to venous stasis
Blood pools in the lower extremities, causing dilation and tortuous veins
Risk factors: Prolonged standing, obesity, pregnancy, and age
Symptoms: Swelling, pain, and visible twisted veins
Venous Thrombosis (Deep Vein Thrombosis - DVT):
Formation of a blood clot (thrombus) in the deep veins, usually in the legs
Can lead to pulmonary embolism if the clot dislodges and travels to the lungs
Risk factors: Immobility, surgery, pregnancy, and genetic clotting disorders
Symptoms: Swelling, pain, and redness in the affected limb
Chronic Venous Insufficiency (CVI):
Long-term impaired venous return leading to chronic swelling, skin changes, and venous ulcers
Often occurs after repeated DVT or in individuals with severe varicose veins
Symptoms: Leg swelling, skin discoloration, pain, and non-healing ulcers
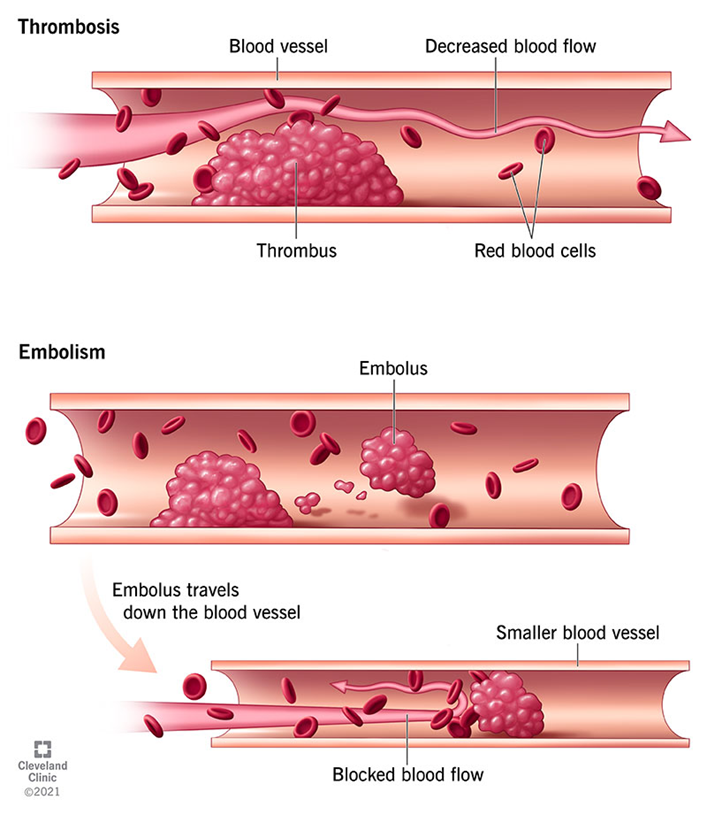
Introduction to Thrombosis and Emboli
Thrombosis:
The formation of a blood clot (thrombus) within an intact blood vessel.
Clots can obstruct blood flow and lead to tissue ischemia.
Virchow's Triad outlines the three main factors contributing to thrombosis:
Endothelial injury: Damage to the blood vessel lining (e.g., due to atherosclerosis, trauma).
Abnormal blood flow: Turbulent or stagnant blood flow (e.g., immobility, aneurysms).
Hypercoagulability: Increased tendency of the blood to clot (e.g., genetic thrombophilias, cancer).
Emboli:
Embolism occurs when a dislodged thrombus (or other material such as fat, air, or tumor cells) travels through the bloodstream and lodges in a distant vessel.
Types of Emboli:
Pulmonary embolism: A thrombus from a deep vein (usually in the leg) travels to the lungs, causing a potentially life-threatening blockage.
Systemic embolism: An embolus from the heart or large arteries lodges in organs like the brain (causing a stroke) or kidneys.
Thrombosis and emboli can lead to life-threatening conditions such as heart attacks, strokes, and pulmonary embolism.
Atherosclerosis is the underlying cause of most cardiovascular diseases, including coronary artery disease and cerebrovascular accidents (strokes).
Thrombosis: Overview and Causes
Definition of Thrombosis:
Thrombosis is the formation of a blood clot within a blood vessel, obstructing the flow of blood and potentially leading to tissue damage or ischemia.
Types of Thrombosis:
Arterial Thrombosis: Occurs in arteries, leading to conditions like heart attacks (myocardial infarction) and strokes.
Venous Thrombosis: Occurs in veins, most commonly in the deep veins of the legs (deep vein thrombosis – DVT), which can lead to pulmonary embolism if the clot travels to the lungs.
Key Causes of Thrombosis:
Endothelial Injury:
Damage to the inner lining of blood vessels (endothelium) is a primary cause of thrombosis.
Atherosclerosis, trauma, surgery, and inflammatory conditions can cause endothelial damage.
Abnormal Blood Flow:
Turbulent flow (in arteries) or stasis (slow, pooled blood in veins) contributes to thrombosis.
Occurs in conditions like immobility, varicose veins, and heart valve disease.
Hypercoagulability:
Increased tendency for blood to clot, which can be due to inherited or acquired conditions.
Genetic mutations, such as Factor V Leiden, and conditions like cancer or pregnancy can contribute
Risk Factors for Thrombosis
Endothelial Injury Risk Factors:
Atherosclerosis: Plaques within arteries cause endothelial damage and increase the risk of arterial thrombosis.
Surgery or Trauma: Disruption of blood vessels during surgery or injury exposes the underlying collagen and tissue factor, leading to clot formation.
Smoking: Promotes endothelial damage, increasing the likelihood of thrombosis.
Abnormal Blood Flow Risk Factors:
Prolonged Immobility:
Inactivity (e.g., during long flights, bed rest, or hospitalization) slows blood flow in the veins, particularly in the legs, increasing the risk of venous thrombosis.
Heart Failure:
Impaired heart function can lead to stasis of blood in the heart or peripheral vessels, promoting clot formation.
Varicose Veins:
Damaged valves in veins cause pooling of blood, contributing to slow flow and increased risk of clot formation.
Genetic Predisposition:
Inherited conditions such as Factor V Leiden mutation or Prothrombin gene mutation (G20210A) increase the risk of abnormal clotting.
Deficiencies in natural anticoagulants such as Protein C, Protein S, or Antithrombin III can also lead to hypercoagulability.
Hormonal Influences:
Use of oral contraceptives or hormone replacement therapy increases the risk of venous thrombosis, particularly in women with other risk factors.
Pregnancy: Increased levels of estrogen during pregnancy promote hypercoagulability to prevent excessive bleeding during childbirth, but this also increases the risk of thrombosis.
Cancer:
Certain cancers (especially pancreatic, lung, and gastrointestinal cancers) can promote hypercoagulability by releasing procoagulant substances into the bloodstream.
Additional Risk Factors:
Obesity: Increased weight is associated with higher levels of inflammatory markers, which can contribute to clot formation.
Age: Risk of thrombosis increases with age due to changes in blood vessel integrity and slower blood flow.
Embolism: Overview and Types
Definition of Embolism:
An embolism occurs when a dislodged substance (typically a thrombus or clot) travels through the bloodstream and becomes lodged in a blood vessel, obstructing blood flow.
Types of Emboli:
Thromboembolism: The most common type, where a blood clot (thrombus) breaks loose and travels through the bloodstream.
Fat Embolism: Occurs when fat droplets enter the bloodstream, usually following long bone fractures or trauma.
Air Embolism: Air bubbles that enter the bloodstream, often due to surgical procedures or trauma.
Amniotic Fluid Embolism: A rare complication of childbirth, where amniotic fluid enters the mother’s circulation.
Septic Embolism: Fragments of infected material (e.g., bacteria) from an infection that travels through the bloodstream, potentially leading to sepsis.
Pulmonary vs. Systemic Embolism:
Pulmonary Embolism (PE): A thrombus, typically from the deep veins of the legs (DVT), travels to the lungs.
Systemic Embolism: An embolus that travels through the arterial system, potentially affecting any organ (e.g., brain, kidneys).
Pulmonary Embolism (PE)
Pulmonary Embolism (PE) Overview:
A life-threatening condition where a clot (typically from a DVT) travels to the pulmonary arteries in the lungs, blocking blood flow.
Causes:
Most commonly results from deep vein thrombosis (DVT) in the legs, which dislodges and travels through the venous system to the lungs.
Symptoms:
Sudden onset of shortness of breath.
Chest pain that worsens with breathing.
Rapid heart rate (tachycardia) and hypotension.
In severe cases, PE can cause cyanosis (bluish discoloration of the skin) and collapse.
Clinical Implications:
Small PEs: May be asymptomatic or cause mild respiratory symptoms but may resolve on their own.
Massive PEs: Can cause acute right heart failure (due to increased pressure in the pulmonary circulation), collapse, and sudden death if not treated promptly.
Diagnosis and Treatment:
CT pulmonary angiography is the gold standard for diagnosing PE.
Treatment includes anticoagulation therapy (heparin, warfarin) and, in severe cases, thrombolytic therapy (clot busters) or surgical intervention.
Systemic Embolism
Systemic Embolism Overview:
An embolus that originates from the left side of the heart or arterial system and travels to organs such as the brain, kidneys, or limbs.
Causes:
Cardiac emboli: The most common source, often arising from atrial fibrillation, mitral valve disease, or after a myocardial infarction.
Atherosclerotic plaque rupture: Plaque fragments can dislodge and become emboli, traveling through the arterial system.
Common Sites of Embolization:
Brain: Leading to ischemic stroke, causing sudden weakness, confusion, or loss of consciousness.
Kidneys: Leading to renal infarction, causing sudden flank pain and hematuria (blood in urine).
Limbs: Can result in acute limb ischemia, causing pain, pallor, and loss of function in the affected limb.
Clinical Implications:
Ischemia and Infarction: Systemic emboli can block blood flow to vital organs, leading to tissue death (infarction).
Stroke Risk: Emboli that travel to the brain can cause strokes, leading to permanent neurological deficits or death if not treated urgently.
Treatment:
Anticoagulation therapy to prevent further emboli.
Thrombolysis or embolectomy to remove the embolus in life-threatening cases.
Clinical Correlations
Raynaud’s Phenomenon:
Physiological Basis: Characterized by excessive vasoconstriction of small arteries and arterioles, typically triggered by cold exposure or emotional stress. This leads to ischemia in the extremities, followed by reactive hyperemia as blood flow returns.
Varicose Veins:
Physiological Basis: Result from venous valve incompetence, which causes venous stasis and increased venous pressure. Over time, this leads to venous dilation, tortuosity, and eventual venous insufficiency, primarily affecting the lower extremities.
Vascular Tumors:
Physiological Basis: Arise from the endothelial cells of blood or lymphatic vessels. These neoplasms can range from benign hemangiomas, which often regress spontaneously, to malignant angiosarcomas, which exhibit aggressive vascular proliferation and potential metastasis.
Explain the physiologic basis of acute disseminated intravascular coagulation and familial hypercholesterolemia.
Shock: Causes and Types
Shock Overview:
Shock occurs when inadequate tissue perfusion leads to poor oxygen delivery, risking organ failure and death.
Types of Shock:
Cardiogenic:
Cause: Heart’s failure to pump effectively (e.g., heart attack).
Symptoms: Low blood pressure, cold skin, weak pulse, JVD, pulmonary edema.
Hypovolemic:
Cause: Fluid loss (e.g., bleeding, dehydration).
Symptoms: Rapid heart rate, low pressure, cool skin, reduced urine output.
Septic:
Cause: Infection causing vasodilation and poor perfusion.
Symptoms: Fever, warm then cold skin, low pressure, confusion.
Obstructive:
Cause: Physical blockage of blood flow (e.g., pulmonary embolism).
Symptoms: Low pressure, JVD, shortness of breath.
Systemic Effects of Shock on Organs
Ischemic Encephalopathy:
Reduced perfusion to the brain leads to confusion, altered mental status, or coma.
Prolonged ischemia can cause neuronal death, resulting in permanent brain damage or death.
Heart:
Myocardial Ischemia:
Decreased blood supply to the heart can worsen pre-existing heart conditions, leading to arrhythmias, myocardial infarction, or cardiogenic shock.
The heart may fail to maintain adequate cardiac output, further exacerbating tissue hypoperfusion.
Lungs:
Acute Respiratory Distress Syndrome (ARDS):
Shock can cause damage to the lung capillaries, leading to leakage of fluid into the alveoli, impeding gas exchange.
Patients may experience hypoxia, respiratory failure, and require mechanical ventilation.
Kidneys:
Acute Tubular Necrosis (ATN):
The kidneys are highly sensitive to ischemia, and reduced blood flow can lead to acute kidney injury (AKI).
If not reversed, this can progress to renal failure, requiring dialysis.
Gastrointestinal Tract:
Ischemic Necrosis:
Reduced perfusion to the intestines can cause ischemic injury to the bowel, leading to bowel infarction and potentially fatal complications like perforation and peritonitis.
Liver:
Centrilobular Necrosis:
The liver’s ability to detoxify and synthesize proteins is compromised due to hypoperfusion, leading to liver failure.
Elevated liver enzymes and jaundice may develop.
Skin:
Pallor and Cold Extremities:
In shock, blood is diverted away from the skin to vital organs, causing cool, clammy skin and pallor.
Pathophysiology of Hemorrhage
Hemorrhage Overview:
Hemorrhage is the escape of blood from a ruptured vessel, leading to blood loss either externally or internally.
Types of Hemorrhage:
Petechiae:
Small (1-2 mm) pinpoint hemorrhages in the skin or mucous membranes, often due to platelet dysfunction or capillary fragility.
Purpura:
Slightly larger (3-5 mm) hemorrhages, commonly seen in conditions like vasculitis or coagulation disorders.
Ecchymoses (Bruises):
Larger hemorrhages (1-2 cm) that appear as skin discolorations due to the breakdown of hemoglobin from red blood cells.
Hemothorax, Hemopericardium, Hemoperitoneum, Hemarthrosis:
Large accumulations of blood in body cavities (e.g., chest, abdomen, joints) resulting from trauma or ruptured blood vessels.
Clinical Implications:
Significant hemorrhage can lead to hypovolemic shock, where the body cannot maintain adequate blood pressure and organ perfusion.
Chronic blood loss can result in iron-deficiency anemia as the body loses red blood cells and iron over time.
Physiology - Week 5 Study Guide: Structure and Function of Blood Vessels and Hemodynamic Disorders
Describe the structure and function of normal blood vessels, including arteries, veins, capillaries, and the relationship to the distribution of blood volume and blood pressure throughout the circulatory system.
Overview of Blood Vessels
Arteries:
Thick walls, high pressure
Types:
Large (elastic) arteries (e.g., aorta, common carotid): lots of elastic fibers, pulsatile flow.
Medium (muscular) arteries (e.g., coronary, renal): mostly smooth muscle cells.
Small arteries/arterioles: primarily smooth muscle cells, regulate blood pressure.
Veins:
Large lumen, low pressure, compressible walls, valves present.
Function: Return blood to the heart under low pressure.
Capillaries:
Very thin walls, large numbers, slow blood flow.
Role: Exchange of oxygen and nutrients with tissues.
Lymphatic System:
Drains excess interstitial fluid, passes through lymph nodes to check for infection.
Overview of Arteries
Large (Elastic) Arteries:
Examples: Aorta, common carotid, iliac arteries.
Contain many elastic fibers, allowing them to stretch and recoil with the heartbeat.
Function: Accommodate high pressure from the heart and maintain continuous blood flow.
Medium (Muscular) Arteries:
Examples: Coronary and renal arteries.
Primarily made of smooth muscle, controlling the diameter and blood flow to organs.
Function: Distribute blood to specific organs.
Small Arteries/Arterioles:
Composed mostly of smooth muscle cells.
Function: Major site of resistance and regulation of blood pressure through vasoconstriction and vasodilation.
Analyze the elasticity of arterioles as well as the function and control of vasoconstriction and vasodilation on blood pressure and the flow of blood.
Elasticity of Arterioles
Regulation of Vascular Tone:
The elasticity of arterioles helps them adjust their diameter through vasoconstriction and vasodilation.
These changes in tone are influenced by:
Autonomic nervous system signals (e.g., sympathetic stimulation causes vasoconstriction).
Hormonal control (e.g., adrenaline and angiotensin cause vasoconstriction, while nitric oxide causes vasodilation).
Impact on Blood Pressure:
Vasoconstriction: The smooth muscle contracts, reducing the diameter of the arteriole, increasing resistance to blood flow, and raising blood pressure.
Vasodilation: The smooth muscle relaxes, increasing the diameter of the arteriole, reducing resistance, and lowering blood pressure.
Loss of Elasticity:
Conditions like atherosclerosis or hypertension can reduce the elasticity of arterioles.
This leads to increased vascular resistance and higher blood pressure, contributing to the development of cardiovascular diseases.
Overview of Veins, Capillaries and Lymphatics
Veins:
Have larger lumens and thinner walls compared to arteries.
Operate under low pressure and have valves to prevent backflow of blood.
Function: Return blood to the heart from the body.
Capillaries:
The smallest blood vessels, approximately the size of a red blood cell in diameter.
Thin-walled and numerous, allowing for the slow exchange of gases, nutrients, and waste.
Function: Facilitate the exchange between blood and tissues.
Lymphatic System:
A network of vessels that collect excess interstitial fluid and return it to the bloodstream.
Function: Maintain fluid balance and immune function by filtering pathogens.
Measurement of Blood Pressure
Systolic Pressure:
The pressure in the arteries when the heart contracts (beats).
Typically the higher number in a blood pressure reading (e.g., 120 in 120/80).
Diastolic Pressure:
The pressure in the arteries when the heart is at rest between beats.
Typically the lower number in a blood pressure reading (e.g., 80 in 120/80).
Korotkoff Sounds:
Sounds heard through a stethoscope while measuring blood pressure using a cuff.
First Korotkoff sound: indicates systolic pressure.
Last Korotkoff sound: indicates diastolic pressure.
Proper Measurement Technique:
Place the cuff on the upper arm and inflate to above systolic pressure.
Slowly deflate while listening for Korotkoff sounds.
Record both the systolic and diastolic pressure accurately.
The Role of Arteries in Blood Pressure Regulation
Elastic Arteries:
Act as pressure reservoirs.
Stretch when blood is ejected from the heart and recoil to maintain blood pressure during heart relaxation (diastole).
Function: Help smooth out the pulsatile nature of blood flow from the heart, maintaining continuous flow.
Muscular Arteries:
Distribute blood to specific organs and regions of the body.
The smooth muscle in their walls can contract or relax to regulate blood flow.
Function: Control blood pressure by adjusting their diameter through vasoconstriction and vasodilation.
Arterioles:
The primary site of resistance in the circulatory system.
Regulate blood flow into capillaries through vasoconstriction (narrowing) and vasodilation (widening).
Function: Control systemic blood pressure by adjusting resistance.
Blood Pressure Control:
Vasoconstriction: Increases resistance, raising blood pressure.
Vasodilation: Decreases resistance, lowering blood pressure.
Arterioles respond to neural signals (from the autonomic nervous system) and hormones (e.g., adrenaline) to regulate blood pressure.
Function of Veins in Blood Volume Regulation
Veins as Blood Reservoirs:
Veins hold about 60-70% of the total blood volume at any given time.
Due to their large lumen and thin walls, veins can stretch to accommodate changes in blood volume without a significant increase in pressure.
Low-Pressure System:
Veins operate under lower pressure than arteries.
Blood returns to the heart via veins through the assistance of valves and skeletal muscle contractions (the muscle pump).
Venous Valves:
Prevent backflow of blood, ensuring it moves in one direction towards the heart.
Important in lower extremities where blood must move against gravity.
Role in Circulation:
Veins adjust to changes in blood volume, helping to regulate cardiac output and blood pressure.
When more blood is needed, veins constrict (venoconstriction) to push blood towards the heart, increasing venous return.
Factors Affecting Venous Return:
Gravity, breathing (thoracic pressure changes), and body position all impact how efficiently veins return blood to the heart.
Structure and Function of Lymphatics
Lymphatic System Overview:
A network of vessels, nodes, and organs that collect excess interstitial fluid and return it to the bloodstream.
Plays a key role in maintaining fluid balance and supporting the immune system.
Function of Lymphatics:
Fluid Return: Collect and return excess interstitial fluid to the bloodstream, preventing edema.
Immune Surveillance: Lymph passes through lymph nodes, where immune cells monitor for infections or abnormalities.
Fat Absorption: Specialized lymphatic vessels called lacteals absorb fats from the digestive system and transport them to the bloodstream.
Lymphatic Vessels:
Similar to veins but thinner walls and more valves.
Begin as blind-ended capillaries in tissues, collecting excess fluid that leaks from blood capillaries.
Lymph Nodes:
Small, bean-shaped structures along lymphatic vessels.
Function: Filter lymph fluid, trapping pathogens, debris, and cancer cells.
Contain immune cells (e.g., lymphocytes) that initiate immune responses when necessary.
Importance in Circulation:
By returning fluid to the bloodstream, the lymphatic system helps maintain blood volume and pressure.
Vessel Structure and Function
Endothelial Cells (ECs): Line blood vessels, controlling blood flow, clotting, and inflammation.
Smooth Muscle Cells (SMCs): Regulate contraction and blood pressure.
Balance: Proper interaction between ECs and SMCs ensures healthy vessel function.
Causes of Vessel Damage
Hypertension: High blood pressure stresses vessel walls, damaging ECs.
Atherosclerosis: Plaque buildup (from fats and cholesterol) narrows arteries.
Inflammation: Diseases like vasculitis cause vessel inflammation and damage.
Toxins: Smoking and other toxic exposures damage vessel linings, accelerating plaque formation.
Physical Injury: Trauma or infections can also harm vessels.
Vessel Response to Injury
Blood Vessel Injury: Trauma, infection, or inflammation disrupts blood flow, often leading to ARTERIOsclerosis (general arterial stiffening).
Arteriosclerosis is a general term that refers to the thickening, hardening, and loss of elasticity in the walls of the arteries
This stiffening of the arteries can reduce blood flow to organs and tissues, increasing the risk of hypertension and other cardiovascular issues
Affects the small arteries and arterioles and is usually associated with aging
Endothelial Cell Loss: Losing ECs triggers repair processes, activating SMCs and the immune system, which can contribute to ATHEROsclerosis (plaque buildup).
Atherosclerosis is a specific type of arteriosclerosis that involves the buildup of plaques (made of fat, cholesterol, and other substances) inside the artery walls
These plaques can narrow the arteries, restrict blood flow, and lead to complications such as heart attacks, strokes, or peripheral artery disease
Commonly affects larger arteries like those in the heart, brain, and legs.
SMC Activation and Intimal Thickening: SMCs migrate to the vessel's inner layer (intima), multiply, and cause thickening—common in atherosclerosis. This can result in stenosis (narrowing), reducing blood flow.
SUMMARY = while ARTERIOsclerosis refers to the general hardening of the arteries, ATHEROsclerosis specifically refers to the plaque buildup that can lead to more serious cardiovascular events.
Consequences of Vessel Damage
Intimal Thickening: SMCs thicken vessel walls, leading to stenosis (narrowing).
Ischemia: Reduced blood flow leads to oxygen deprivation in tissues.
Clinical Effects:
Heart: Stenosis can cause angina or myocardial infarction.
Brain: Narrowed carotid arteries increase stroke risk.
Limbs: Reduced flow can result in gangrene or severe pain.
ATHEROsclerosis
Atherosclerosis Overview:
A chronic condition characterized by the buildup of plaque (lipids, cholesterol, calcium, and other substances) in the inner walls of arteries.
Plaques narrow the arteries, reducing blood flow and increasing the risk of blockages.
Development of Atherosclerosis:
Begins with endothelial injury, which leads to inflammation and the accumulation of lipids.
Over time, plaques grow larger, causing luminal stenosis.
Rupture of plaques can lead to the formation of blood clots (thrombosis), which can obstruct the artery entirely.
Risk Factors:
High cholesterol, smoking, hypertension, diabetes, and a sedentary lifestyle increase the risk of atherosclerosis.
Family history and aging are also significant factors.
Modifiable vs. nonmodifiable risk factors
Clinical Consequences:
Coronary artery disease: Atherosclerosis in the coronary arteries can cause angina or myocardial infarction (heart attack).
Cerebrovascular disease: Plaque buildup in the carotid arteries can lead to stroke.
Peripheral artery disease: Affects blood flow to the extremities, causing pain and tissue damage.
ATHEROsclerosis Modifiable Risk Factors
Hyperlipidemia:
Elevated levels of LDL cholesterol are a major risk factor for atherosclerosis.
HDL cholesterol helps remove cholesterol from plaques, reducing risk.
Hypertension:
High blood pressure damages the endothelium, accelerating plaque formation.
Smoking:
Damages the endothelium and increases oxidative stress and inflammation, promoting plaque development.
Diabetes:
High blood sugar levels lead to endothelial dysfunction and increased lipid deposition in arteries.
Obesity and Physical Inactivity:
Both are linked to metabolic disturbances that increase the risk of developing atherosclerosis.
ATHEROsclerosis NON-Modifiable Risk Factors
Age:
The risk of atherosclerosis increases with age.
Gender:
Men are at higher risk than premenopausal women, though the risk increases in women after menopause.
Family History:
A genetic predisposition to cardiovascular disease increases the risk of developing atherosclerosis.
ATHEROsclerosis Additional Risk Factors
C-reactive protein (CRP):
Elevated CRP levels indicate systemic inflammation, which is linked to increased atherosclerosis risk.
Sedentary Lifestyle:
Lack of regular physical activity contributes to several modifiable risk factors, including obesity and hypertension.
Other Common Vascular Diseases: Dissections
Aortic Dissection:
Occurs when blood enters the media layer of the aortic wall, separating the layers and creating a false lumen.
Type A Dissection
Involves the ascending aorta and is more dangerous; requires immediate surgery.
Type B Dissection
Involves the descending aorta and may be managed with medication if stable.
Risk Factors for Aneurysms and Dissections:
Hypertension: Increases the risk of both aneurysms and dissections.
Connective tissue disorders (e.g., Marfan syndrome) also predispose individuals to these conditions.
Atherosclerosis: Aneurysms are often associated with underlying atherosclerosis
Other Common Vascular Diseases: Aneurysms
Aneurysms:
A localized dilation or bulging of a blood vessel wall, usually due to a weakness in the vessel wall.
Commonly occurs in the aorta (abdominal aortic aneurysm) or cerebral arteries.
Types:
True Aneurysm:
Involves all three layers of the arterial wall (e.g., fusiform or saccular).
False Aneurysm:
A breach in the vessel wall leads to a collection of blood outside the vessel, contained by surrounding tissue.
Clinical Consequences of Aneurysms:
Rupture
Can cause life-threatening hemorrhage. For example, a ruptured aortic aneurysm can lead to sudden death.
Compression of nearby structures
Large aneurysms may compress organs or nerves.
Thrombus formation
Blood clots may form in the dilated area, leading to embolization.
Aneurysm vs Dissection
Aneurysm
A localized bulge in the vessel due to wall weakening.
Dissection
A tear in the inner layer of the artery, leading to separation between the vessel layers
Both conditions are dangerous, but their underlying mechanisms differ—aneurysms involve dilation, while dissections involve a tear and separation within the vessel wall.
Describe the distribution of fluid between the intracellular and extracellular compartments and identify the basic aspects of normal circulation.
Intracellular Fluid (ICF):
Location: Inside cells, makes up two-thirds of body fluid.
Major Ions: Potassium (K⁺) and Phosphate (PO₄³⁻).
Functions:
Maintains cell shape, supports metabolism.
Regulates osmotic balance and nutrient/waste exchange.
Extracellular Fluid (ECF):
Location: Outside cells, one-third of body fluid.
Components:
Interstitial Fluid (around cells).
Plasma (blood component).
Major Ions: Sodium (Na⁺) and Chloride (Cl⁻).
Functions:
Transports nutrients, oxygen, and waste.
Regulates blood pressure and osmotic pressure.
Capillary Exchange Mechanisms (Review)
Capillary Exchange Mechanisms:
Forces Driving Fluid Movement:
Hydrostatic Pressure: Pushes fluid out of capillaries.
Osmotic (Oncotic) Pressure: Pulls fluid into capillaries via plasma proteins (albumin).
Balance:
Filtration at arterial end (hydrostatic > osmotic).
Reabsorption at venous end (osmotic > hydrostatic).
Lymphatic System: Drains excess fluid, preventing edema
Edema: Causes and Types (Transudate vs. Exudate)
Definition of Edema:
Edema is the accumulation of excess fluid in the interstitial tissue spaces, leading to swelling.
Causes of Edema:
Increased Hydrostatic Pressure:
Often due to impaired venous return (e.g., heart failure, deep vein thrombosis), causing fluid to be pushed out of capillaries into the interstitial space.
Decreased Plasma Oncotic Pressure:
Caused by a reduction in plasma proteins (e.g., hypoalbuminemia from liver disease or nephrotic syndrome), leading to less fluid being reabsorbed into capillaries.
Lymphatic Obstruction:
Impaired lymphatic drainage (e.g., from cancer, surgery, or infections) results in fluid accumulation in tissues.
Increased Vascular Permeability:
Inflammatory conditions (e.g., infections, allergic reactions) increase the permeability of capillaries, allowing fluid and proteins to leak into the interstitial space.
Sodium and Water Retention:
Disorders such as renal failure can lead to excessive sodium retention, increasing blood volume and pressure, promoting edema.
Transudate:
Protein-poor fluid caused by imbalances in hydrostatic or oncotic pressure.
Typically associated with non-inflammatory conditions like heart failure or liver disease.
Fluid is clear and low in cells.
Exudate:
Protein-rich fluid caused by increased capillary permeability, often in response to inflammation.
Associated with conditions such as infections, trauma, or malignancy.
Fluid is cloudy and contains immune cells, proteins, and other debris.
Pathophysiology of Hyperemia
Hyperemia Overview:
Hyperemia refers to an increase in blood flow to a particular tissue, resulting in redness and warmth in the affected area.
Types of Hyperemia:
Active Hyperemia:
Caused by arteriolar dilation due to increased tissue demand for oxygen (e.g., during exercise or inflammation).
Blood vessels dilate to increase the flow of oxygenated blood to the tissues.
Passive Hyperemia (Congestion):
Caused by impaired venous outflow, leading to an accumulation of deoxygenated blood in the tissues (e.g., in heart failure or venous obstruction).
Affected tissues appear blue or cyanotic due to low oxygen levels.
Clinical Relevance:
Acute hyperemia can occur in response to injury or inflammation, while chronic congestion (e.g., in liver cirrhosis or chronic heart failure) can lead to tissue damage and fibrosis over time.
Role of Lymphatic Drainage in Fluid Regulation
Lymphatic System Overview:
A network of vessels that collects excess interstitial fluid and returns it to the bloodstream.
Acts as a safety valve to prevent the accumulation of fluid in tissues.
Lymphatic Capillaries:
Blind-ended, thin-walled structures that are highly permeable to proteins and larger molecules that cannot reenter capillaries.
Drain excess fluid from the interstitial space, transporting it through lymph nodes for immune surveillance.
Prevention of Edema:
The lymphatic system helps maintain fluid balance by removing any excess fluid that remains after capillary filtration and reabsorption.
If lymphatic drainage is impaired (e.g., due to blockage or removal of lymph nodes), lymphedema can develop.
Immune Function:
Lymph nodes along the lymphatic vessels filter fluid, trapping pathogens, and debris.
Plays a crucial role in initiating immune responses by activating lymphocytes when pathogens are detected.
Return to Circulation:
The lymphatic fluid, now called lymph, is eventually returned to the bloodstream via the thoracic duct, maintaining blood volume and pressure.
Explain normal hemostasis and the role of endothelial cells, platelets, and the coagulation proteins in the extrinsic and intrinsic clotting cascade along with associated dysfunction.
Normal Hemostasis Process: Vasoconstriction and Platelet Plug Formation
Hemostasis Overview:
Hemostasis is the body’s natural process of stopping bleeding from an injured vessel, ensuring proper clot formation while maintaining blood flow.
Step 1: Vasoconstriction:
Vascular spasm occurs immediately after vessel injury to reduce blood flow and limit blood loss.
Triggered by local nervous reflexes and the release of endothelin, a vasoconstrictor released by endothelial cells.
Temporary, but crucial in allowing time for other hemostatic processes to activate.
Step 2: Platelet Plug Formation (Primary Hemostasis):
Platelets adhere to the exposed collagen in the damaged vessel wall, facilitated by von Willebrand factor (vWF).
Platelets become activated and change shape, releasing chemicals like ADP and Thromboxane A2, which attract more platelets to the site.
Platelets aggregate to form a primary hemostatic plug, temporarily sealing the injury.
Platelet Activation:
Platelets release granules containing clotting factors that further enhance aggregation and strengthen the platelet plug.
This plug is fragile and needs to be stabilized by the coagulation process
Normal Hemostasis Process: Coagulation (Secondary Homeostasis)
Step 3: Coagulation Cascade:
Secondary hemostasis stabilizes the platelet plug through the formation of a fibrin clot.
Involves a series of enzymatic reactions that activate clotting factors (e.g., Factors VII, X, and thrombin).
The cascade is divided into two pathways:
Intrinsic Pathway: Activated by trauma inside the blood vessel, involving Factors XII, XI, IX, and VIII.
Extrinsic Pathway: Activated by external trauma, involving Tissue Factor (TF) and Factor VII.
Both pathways converge at the common pathway, where Factor X is activated, leading to the conversion of prothrombin to thrombin.
Formation of Fibrin Mesh:
Thrombin converts fibrinogen (soluble) into fibrin (insoluble), which forms a mesh that strengthens and stabilizes the platelet plug.
The result is a durable secondary hemostatic plug that effectively stops bleeding.
Clot Retraction and Dissolution:
After the clot forms, platelets contract, pulling the edges of the wound closer together (clot retraction).
Eventually, the clot is dissolved by plasmin in a process called fibrinolysis, restoring normal blood flow after healing.
Role of Endothelial Cells in Hemostasis
Endothelial Cells Overview:
Endothelial cells (ECs) line the interior surface of blood vessels and play a key role in maintaining vascular integrity.
ECs act as a barrier between the blood and the vessel wall, regulating blood flow and preventing abnormal clot formation.
Antithrombotic Properties of Endothelial Cells:
Antiplatelet Effects:
Intact ECs produce prostacyclin (PGI2) and nitric oxide (NO), which inhibit platelet aggregation and promote vasodilation, preventing unnecessary clotting.
ECs also release adenosine diphosphatase, which degrades ADP to inhibit platelet adhesion.
Anticoagulant Properties:
ECs express heparin-like molecules that enhance the activity of antithrombin, a natural anticoagulant that inactivates thrombin and other clotting factors.
ECs also produce thrombomodulin, which binds thrombin and activates protein C, a key anticoagulant that degrades Factors Va and VIIIa.
Fibrinolytic Properties:
ECs produce tissue plasminogen activator (t-PA), which converts plasminogen to plasmin, dissolving clots after they have formed and ensuring blood flow is restored.
The Coagulation Cascade: Overview
Coagulation Cascade Overview:
The coagulation cascade is a series of enzymatic reactions that lead to the formation of a fibrin clot, stabilizing the platelet plug and stopping bleeding.
It involves two main pathways:
Intrinsic Pathway: Activated by damage inside the blood vessel.
Extrinsic Pathway: Activated by external trauma to the vessel.
Both pathways converge at the common pathway, leading to the production of thrombin and fibrin.
Key Players:
Clotting Factors: These are proteins (e.g., Factor I, II, V, VII, VIII, IX, X) that are activated sequentially in a cascade.
Calcium and phospholipids are essential cofactors in the activation of clotting factors.
The Intrinsic Pathway
Activation of Intrinsic Pathway:
Triggered by damage to the blood vessel itself (e.g., exposure of collagen or abnormal blood flow).
Involves a series of clotting factors (XII, XI, IX, and VIII) that are activated in sequence.
Key Steps:
Factor XII is activated upon contact with exposed collagen or a negatively charged surface.
Activated Factor XII (XIIa) converts Factor XI to its active form (XIa).
Factor XIa activates Factor IX to IXa.
Factor IXa, in the presence of Factor VIIIa and calcium, activates Factor X, beginning the common pathway.
Clinical Relevance:
Disorders of the intrinsic pathway (e.g., hemophilia A and B) result from deficiencies in Factor VIII or Factor IX, leading to impaired clot formation and excessive bleeding.
The Extrinsic Pathway and Common Pathway
Activation of Extrinsic Pathway:
Initiated by tissue factor (TF), a protein expressed on the surface of cells outside the blood vessel in response to external trauma.
TF binds to Factor VII, forming a complex that activates Factor VIIa.
This complex directly activates Factor X, leading to the common pathway.
Common Pathway:
Activated Factor X (Xa) converts prothrombin (Factor II) to thrombin in the presence of Factor Va, calcium, and phospholipids.
Thrombin converts fibrinogen (Factor I) into fibrin, which forms a mesh to stabilize the platelet plug.
Thrombin also activates Factor XIII, which cross-links fibrin strands, further stabilizing the clot.
Regulation and Control:
Natural anticoagulants (e.g., antithrombin, protein C, and protein S) ensure that the coagulation cascade is localized and does not lead to excessive clotting.
Clinical Relevance:
Disorders of the extrinsic pathway (e.g., Factor VII deficiency) result in impaired clotting after trauma.
The Prothrombin Time (PT) test assesses the extrinsic and common pathways, while the Activated Partial Thromboplastin Time (aPTT) test evaluates the intrinsic pathway.
Clot Retraction
Clot Retraction Overview:
Clot retraction is the process where the fibrin mesh contracts, pulling the edges of the wound closer together.
Begins shortly after the clot forms, typically within minutes, and continues for hours.
Mechanism of Clot Retraction:
Platelets trapped within the clot contain actin and myosin, similar to muscle cells.
These platelets contract, pulling on the fibrin strands, causing the clot to shrink and compress.
This helps reduce the size of the wound and minimize blood loss.
Function:
Reduces the wound gap: Facilitates faster tissue repair and healing by bringing the edges of the injury closer together.
Strengthens the clot: Compacts the clot, making it more resistant to further disruption.
Clinical Relevance:
Impaired clot retraction can indicate platelet function disorders, such as Glanzmann thrombasthenia, where platelets fail to properly bind to fibrin and cause retraction.
Clot Dissolution: Plasminogen Activation and Fibrinolysis
Clot Dissolution (Fibrinolysis):
Once the vessel has healed, the body dissolves the clot through the process of fibrinolysis.
This prevents clots from persisting unnecessarily and obstructing blood flow.
Plasminogen Activation:
Plasminogen is an inactive enzyme that is incorporated into the clot during formation.
It is activated by tissue plasminogen activator (t-PA), which is secreted by endothelial cells, and by urokinase.
When activated, plasminogen is converted into plasmin, the enzyme responsible for breaking down fibrin.
Fibrin Breakdown:
Plasmin cleaves fibrin strands, breaking down the clot into soluble fibrin degradation products (FDPs), which are removed from circulation.
This process restores normal blood flow through the vessel.
Regulation of Fibrinolysis:
Plasminogen activator inhibitors (PAIs) prevent excessive plasmin activity, ensuring that fibrinolysis only occurs when the vessel has healed.
α2-antiplasmin is a major inhibitor of plasmin, regulating its activity to prevent unwarranted clot breakdown.
Clinical Relevance:
Impaired fibrinolysis can result in thrombosis, while excessive fibrinolysis can lead to bleeding disorders.
Therapeutically, t-PA is used as a clot-busting drug to treat conditions like myocardial infarction and stroke by promoting fibrinolysis.
Define and describe hypertension, edema, thrombosis, emboli, atherosclerosis.
Vasoconstriction and Vasodilation Mechanisms
Vasoconstriction Mechanisms
Vasoconstriction:
Definition: The narrowing of blood vessels due to the contraction of smooth muscle in the arteriole walls.
Mechanism:
Triggered by sympathetic nervous system activation.
Hormones like adrenaline, angiotensin II, and vasopressin bind to receptors on smooth muscle cells, causing them to contract.
Increases resistance to blood flow, raising blood pressure.
Function: Helps redirect blood flow to essential organs (e.g., heart and brain) during stress or low blood volume.
Neurotransmitters, Hormones, and Receptors in VasoCONSTRICTION
Vasoconstriction:
Neurotransmitters & Hormones:
Norepinephrine
Binds to alpha-1 adrenergic receptors, causing vessel constriction.
Epinephrine
Binds to alpha-1 receptors for constriction, and beta-2 receptors for dilation in muscles.
Angiotensin II
Activates AT1 receptors, causing potent vasoconstriction.
Vasopressin (ADH)
Binds to V1 receptors on smooth muscle, increasing constriction.
Key Receptors:
Alpha-1 Adrenergic: Norepinephrine/Epinephrine → Constriction.
AT1 Receptors: Angiotensin II → Constriction.
V1 Receptors: Vasopressin → Constriction.
VasoDILATION Mechanisms
Vasodilation:
Definition: The widening of blood vessels due to the relaxation of smooth muscle in the arteriole walls.
Mechanism:
Triggered by the parasympathetic nervous system and certain hormones (e.g., nitric oxide, bradykinin).
Nitric oxide (NO) is a major vasodilator released by endothelial cells, causing smooth muscle relaxation.
Decreases resistance to blood flow, lowering blood pressure.
Function: Increases blood flow to tissues, especially during rest, digestion, or exercise.
Neurotransmitters, Hormones, and Receptors in VasoDILATION
Vasodilation:
Neurotransmitters & Hormones:
Nitric Oxide (NO): Triggers cGMP in smooth muscle → Relaxation.
Acetylcholine (ACh): Stimulates NO release via muscarinic receptors.
Bradykinin: Activates B2 receptors to release NO.
Atrial Natriuretic Peptide (ANP): Binds to NPR receptors → cGMP → Dilation.
Key Receptors:
Beta-2 Adrenergic: Epinephrine → Dilation (muscle vessels).
M3 Muscarinic: ACh → NO release → Dilation.
B2 Receptors: Bradykinin → NO release → Dilation.
NPR Receptors: ANP → cGMP → Dilation.
Balancing Neurotransmitters and Hormones for Homeostasis
Balance & Homeostasis:
Sympathetic system → vasoconstriction (stress, blood pressure).
Parasympathetic system → vasodilation (relaxation, digestion).
Endothelium regulates balance via NO and other factors to maintain blood flow.
Understanding Systolic and Diastolic Pressure
Systolic Pressure:
The pressure in the arteries when the heart contracts and pumps blood into the aorta.
Represents the maximum pressure exerted on the arterial walls during a heartbeat.
Typically the higher number in a blood pressure reading (e.g., 120 mmHg in 120/80).
Important for assessing the force of blood flow and the workload on the heart.
Diastolic Pressure:
The pressure in the arteries when the heart is at rest between beats.
Represents the minimum pressure exerted on the arterial walls.
Typically the lower number in a blood pressure reading (e.g., 80 mmHg in 120/80).
Reflects how well the arteries maintain pressure during the heart’s relaxation phase.
Significance of Both Pressures:
Both systolic and diastolic pressure provide key information about the health of the cardiovascular system.
Elevated systolic pressure can indicate stiffening of arteries or high cardiac output, increasing the risk of heart attack or stroke.
Elevated diastolic pressure often reflects vascular resistance and may point to hypertension-related complications.
Normal vs. Abnormal Readings:
Normal Range: Systolic <120 mmHg, Diastolic <80 mmHg.
Hypertension: Systolic ≥130 mmHg or Diastolic ≥80 mmHg (depending on guidelines and age).
Clinical Relevance:
Monitoring both systolic and diastolic pressures helps detect hypertension, hypotension, and cardiovascular risk, guiding appropriate medical intervention.
Pathology of Hypertension: Overview
What is Hypertension?
Defined as persistently elevated blood pressure. Common thresholds:
Systolic ≥130 mmHg or Diastolic ≥80 mmHg.
Two main types:
Primary (Essential) Hypertension: No identifiable cause, accounting for 90-95% of cases.
Secondary Hypertension: Resulting from an identifiable cause, such as kidney disease or adrenal gland disorders.
Primary Hypertension:
Risk factors include:
Genetic predisposition.
Diet: High sodium intake.
Lifestyle factors: Lack of exercise, obesity, smoking, and stress.
Age and gender: More common with advancing age and in males up to age 65.
Secondary Hypertension:
Caused by:
Kidney disease.
Endocrine disorders (e.g., hyperaldosteronism, pheochromocytoma).
Medications: Certain drugs like NSAIDs or oral contraceptives.
Sleep apnea.
Mechanisms and Effects of Hypertension
Pathophysiology of Hypertension:
Vascular resistance: Hypertension results from increased systemic vascular resistance.
Vessel damage: Chronic high blood pressure damages arterial walls, leading to endothelial dysfunction and vessel thickening.
Imbalance in regulatory mechanisms: Dysregulation of the renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system contributes to increased vascular tone and blood volume.
End-Organ Damage:
Heart:
Hypertension increases the workload on the heart, leading to left ventricular hypertrophy (LVH) and increasing the risk of heart failure.
Coronary artery disease (CAD) is common due to damage to arterial walls.
Brain:
High blood pressure increases the risk of stroke and cerebral hemorrhage due to weakened blood vessels.
Kidneys:
Hypertension damages renal arteries, reducing kidney function and potentially leading to chronic kidney disease (CKD).
Eyes:
Damage to retinal blood vessels can lead to hypertensive retinopathy and vision loss.
A Brief Introduction to Clinical Management of Hypertension
Lifestyle Modifications:
Dietary changes: Reduce sodium intake, increase potassium, and adopt the DASH diet (Dietary Approaches to Stop Hypertension).
Physical activity: Regular exercise helps reduce blood pressure.
Weight management: Achieving and maintaining a healthy weight is key in managing hypertension.
Stress reduction: Managing stress through relaxation techniques, such as meditation, can lower blood pressure.
Pharmacological Treatment:
Diuretics: Help eliminate excess sodium and fluid from the body, lowering blood pressure.
ACE inhibitors/ARBs: Block the effects of angiotensin, reducing vasoconstriction and lowering blood pressure.
Calcium channel blockers: Relax blood vessels by preventing calcium from entering the muscle cells of the heart and arteries.
Beta-blockers: Reduce heart rate and the force of contraction, lowering blood pressure.
Monitoring and Compliance:
Regular blood pressure monitoring is crucial for managing the condition.
Adherence to medication and lifestyle modifications is essential for reducing the risk of complications.
Discuss the pathology and clinical implications behind hemophilia, thalassemia, thrombophilia, polycythemia, hemochromatosis, Raynaud phenomenon, varicose veins, vascular aneurysms, and dissections.
Pathology of Clotting Disorders: Hemophilia
A genetic disorder that results in deficient or defective clotting factors, leading to excessive bleeding.
Types:
Hemophilia A: Caused by a deficiency of Factor VIII.
Hemophilia B: Caused by a deficiency of Factor IX.
Symptoms:
Prolonged bleeding, especially after injury or surgery.
Spontaneous bleeding in joints (hemarthrosis) and muscles.
Treatment:
Replacement therapy with recombinant or plasma-derived Factor VIII or Factor IX.
In severe cases, regular prophylactic factor infusions are needed to prevent bleeding episodes.
Pathology of Clotting Disorders: Thrombophilia
A condition where the blood has an increased tendency to form clots (hypercoagulability), leading to an elevated risk of thrombosis.
Inherited Thrombophilia:
Factor V Leiden mutation: A genetic mutation that makes Factor V resistant to degradation by Protein C, increasing the risk of deep vein thrombosis (DVT).
Prothrombin gene mutation (G20210A): Increases prothrombin levels, enhancing the tendency to form clots.
Acquired Thrombophilia:
Conditions like antiphospholipid syndrome (APS), which promotes clotting through the production of antibodies that target phospholipid-binding proteins.
Prolonged immobility, cancer, pregnancy, or use of oral contraceptives can increase the risk of thrombophilia.
Symptoms:
Recurrent deep vein thrombosis (DVT), pulmonary embolism, or other thrombotic events.
Treatment:
Anticoagulants like warfarin, heparin, or direct oral anticoagulants (DOACs) are commonly used to prevent clot formation
Pathophysiology of Veins
Varicose Veins:
Caused by venous valve incompetence, leading to venous stasis
Blood pools in the lower extremities, causing dilation and tortuous veins
Risk factors: Prolonged standing, obesity, pregnancy, and age
Symptoms: Swelling, pain, and visible twisted veins
Venous Thrombosis (Deep Vein Thrombosis - DVT):
Formation of a blood clot (thrombus) in the deep veins, usually in the legs
Can lead to pulmonary embolism if the clot dislodges and travels to the lungs
Risk factors: Immobility, surgery, pregnancy, and genetic clotting disorders
Symptoms: Swelling, pain, and redness in the affected limb
Chronic Venous Insufficiency (CVI):
Long-term impaired venous return leading to chronic swelling, skin changes, and venous ulcers
Often occurs after repeated DVT or in individuals with severe varicose veins
Symptoms: Leg swelling, skin discoloration, pain, and non-healing ulcers
Introduction to Thrombosis and Emboli
Thrombosis:
The formation of a blood clot (thrombus) within an intact blood vessel.
Clots can obstruct blood flow and lead to tissue ischemia.
Virchow's Triad outlines the three main factors contributing to thrombosis:
Endothelial injury: Damage to the blood vessel lining (e.g., due to atherosclerosis, trauma).
Abnormal blood flow: Turbulent or stagnant blood flow (e.g., immobility, aneurysms).
Hypercoagulability: Increased tendency of the blood to clot (e.g., genetic thrombophilias, cancer).
Emboli:
Embolism occurs when a dislodged thrombus (or other material such as fat, air, or tumor cells) travels through the bloodstream and lodges in a distant vessel.
Types of Emboli:
Pulmonary embolism: A thrombus from a deep vein (usually in the leg) travels to the lungs, causing a potentially life-threatening blockage.
Systemic embolism: An embolus from the heart or large arteries lodges in organs like the brain (causing a stroke) or kidneys.
Thrombosis and emboli can lead to life-threatening conditions such as heart attacks, strokes, and pulmonary embolism.
Atherosclerosis is the underlying cause of most cardiovascular diseases, including coronary artery disease and cerebrovascular accidents (strokes).
Thrombosis: Overview and Causes
Definition of Thrombosis:
Thrombosis is the formation of a blood clot within a blood vessel, obstructing the flow of blood and potentially leading to tissue damage or ischemia.
Types of Thrombosis:
Arterial Thrombosis: Occurs in arteries, leading to conditions like heart attacks (myocardial infarction) and strokes.
Venous Thrombosis: Occurs in veins, most commonly in the deep veins of the legs (deep vein thrombosis – DVT), which can lead to pulmonary embolism if the clot travels to the lungs.
Key Causes of Thrombosis:
Endothelial Injury:
Damage to the inner lining of blood vessels (endothelium) is a primary cause of thrombosis.
Atherosclerosis, trauma, surgery, and inflammatory conditions can cause endothelial damage.
Abnormal Blood Flow:
Turbulent flow (in arteries) or stasis (slow, pooled blood in veins) contributes to thrombosis.
Occurs in conditions like immobility, varicose veins, and heart valve disease.
Hypercoagulability:
Increased tendency for blood to clot, which can be due to inherited or acquired conditions.
Genetic mutations, such as Factor V Leiden, and conditions like cancer or pregnancy can contribute
Risk Factors for Thrombosis
Endothelial Injury Risk Factors:
Atherosclerosis: Plaques within arteries cause endothelial damage and increase the risk of arterial thrombosis.
Surgery or Trauma: Disruption of blood vessels during surgery or injury exposes the underlying collagen and tissue factor, leading to clot formation.
Smoking: Promotes endothelial damage, increasing the likelihood of thrombosis.
Abnormal Blood Flow Risk Factors:
Prolonged Immobility:
Inactivity (e.g., during long flights, bed rest, or hospitalization) slows blood flow in the veins, particularly in the legs, increasing the risk of venous thrombosis.
Heart Failure:
Impaired heart function can lead to stasis of blood in the heart or peripheral vessels, promoting clot formation.
Varicose Veins:
Damaged valves in veins cause pooling of blood, contributing to slow flow and increased risk of clot formation.
Genetic Predisposition:
Inherited conditions such as Factor V Leiden mutation or Prothrombin gene mutation (G20210A) increase the risk of abnormal clotting.
Deficiencies in natural anticoagulants such as Protein C, Protein S, or Antithrombin III can also lead to hypercoagulability.
Hormonal Influences:
Use of oral contraceptives or hormone replacement therapy increases the risk of venous thrombosis, particularly in women with other risk factors.
Pregnancy: Increased levels of estrogen during pregnancy promote hypercoagulability to prevent excessive bleeding during childbirth, but this also increases the risk of thrombosis.
Cancer:
Certain cancers (especially pancreatic, lung, and gastrointestinal cancers) can promote hypercoagulability by releasing procoagulant substances into the bloodstream.
Additional Risk Factors:
Obesity: Increased weight is associated with higher levels of inflammatory markers, which can contribute to clot formation.
Age: Risk of thrombosis increases with age due to changes in blood vessel integrity and slower blood flow.
Embolism: Overview and Types
Definition of Embolism:
An embolism occurs when a dislodged substance (typically a thrombus or clot) travels through the bloodstream and becomes lodged in a blood vessel, obstructing blood flow.
Types of Emboli:
Thromboembolism: The most common type, where a blood clot (thrombus) breaks loose and travels through the bloodstream.
Fat Embolism: Occurs when fat droplets enter the bloodstream, usually following long bone fractures or trauma.
Air Embolism: Air bubbles that enter the bloodstream, often due to surgical procedures or trauma.
Amniotic Fluid Embolism: A rare complication of childbirth, where amniotic fluid enters the mother’s circulation.
Septic Embolism: Fragments of infected material (e.g., bacteria) from an infection that travels through the bloodstream, potentially leading to sepsis.
Pulmonary vs. Systemic Embolism:
Pulmonary Embolism (PE): A thrombus, typically from the deep veins of the legs (DVT), travels to the lungs.
Systemic Embolism: An embolus that travels through the arterial system, potentially affecting any organ (e.g., brain, kidneys).
Pulmonary Embolism (PE)
Pulmonary Embolism (PE) Overview:
A life-threatening condition where a clot (typically from a DVT) travels to the pulmonary arteries in the lungs, blocking blood flow.
Causes:
Most commonly results from deep vein thrombosis (DVT) in the legs, which dislodges and travels through the venous system to the lungs.
Symptoms:
Sudden onset of shortness of breath.
Chest pain that worsens with breathing.
Rapid heart rate (tachycardia) and hypotension.
In severe cases, PE can cause cyanosis (bluish discoloration of the skin) and collapse.
Clinical Implications:
Small PEs: May be asymptomatic or cause mild respiratory symptoms but may resolve on their own.
Massive PEs: Can cause acute right heart failure (due to increased pressure in the pulmonary circulation), collapse, and sudden death if not treated promptly.
Diagnosis and Treatment:
CT pulmonary angiography is the gold standard for diagnosing PE.
Treatment includes anticoagulation therapy (heparin, warfarin) and, in severe cases, thrombolytic therapy (clot busters) or surgical intervention.
Systemic Embolism
Systemic Embolism Overview:
An embolus that originates from the left side of the heart or arterial system and travels to organs such as the brain, kidneys, or limbs.
Causes:
Cardiac emboli: The most common source, often arising from atrial fibrillation, mitral valve disease, or after a myocardial infarction.
Atherosclerotic plaque rupture: Plaque fragments can dislodge and become emboli, traveling through the arterial system.
Common Sites of Embolization:
Brain: Leading to ischemic stroke, causing sudden weakness, confusion, or loss of consciousness.
Kidneys: Leading to renal infarction, causing sudden flank pain and hematuria (blood in urine).
Limbs: Can result in acute limb ischemia, causing pain, pallor, and loss of function in the affected limb.
Clinical Implications:
Ischemia and Infarction: Systemic emboli can block blood flow to vital organs, leading to tissue death (infarction).
Stroke Risk: Emboli that travel to the brain can cause strokes, leading to permanent neurological deficits or death if not treated urgently.
Treatment:
Anticoagulation therapy to prevent further emboli.
Thrombolysis or embolectomy to remove the embolus in life-threatening cases.

Clinical Correlations
Raynaud’s Phenomenon:
Physiological Basis: Characterized by excessive vasoconstriction of small arteries and arterioles, typically triggered by cold exposure or emotional stress. This leads to ischemia in the extremities, followed by reactive hyperemia as blood flow returns.
Varicose Veins:
Physiological Basis: Result from venous valve incompetence, which causes venous stasis and increased venous pressure. Over time, this leads to venous dilation, tortuosity, and eventual venous insufficiency, primarily affecting the lower extremities.
Vascular Tumors:
Physiological Basis: Arise from the endothelial cells of blood or lymphatic vessels. These neoplasms can range from benign hemangiomas, which often regress spontaneously, to malignant angiosarcomas, which exhibit aggressive vascular proliferation and potential metastasis.
Explain the physiologic basis of acute disseminated intravascular coagulation and familial hypercholesterolemia.
Shock: Causes and Types
Shock Overview:
Shock occurs when inadequate tissue perfusion leads to poor oxygen delivery, risking organ failure and death.
Types of Shock:
Cardiogenic:
Cause: Heart’s failure to pump effectively (e.g., heart attack).
Symptoms: Low blood pressure, cold skin, weak pulse, JVD, pulmonary edema.
Hypovolemic:
Cause: Fluid loss (e.g., bleeding, dehydration).
Symptoms: Rapid heart rate, low pressure, cool skin, reduced urine output.
Septic:
Cause: Infection causing vasodilation and poor perfusion.
Symptoms: Fever, warm then cold skin, low pressure, confusion.
Obstructive:
Cause: Physical blockage of blood flow (e.g., pulmonary embolism).
Symptoms: Low pressure, JVD, shortness of breath.
Systemic Effects of Shock on Organs
Ischemic Encephalopathy:
Reduced perfusion to the brain leads to confusion, altered mental status, or coma.
Prolonged ischemia can cause neuronal death, resulting in permanent brain damage or death.
Heart:
Myocardial Ischemia:
Decreased blood supply to the heart can worsen pre-existing heart conditions, leading to arrhythmias, myocardial infarction, or cardiogenic shock.
The heart may fail to maintain adequate cardiac output, further exacerbating tissue hypoperfusion.
Lungs:
Acute Respiratory Distress Syndrome (ARDS):
Shock can cause damage to the lung capillaries, leading to leakage of fluid into the alveoli, impeding gas exchange.
Patients may experience hypoxia, respiratory failure, and require mechanical ventilation.
Kidneys:
Acute Tubular Necrosis (ATN):
The kidneys are highly sensitive to ischemia, and reduced blood flow can lead to acute kidney injury (AKI).
If not reversed, this can progress to renal failure, requiring dialysis.
Gastrointestinal Tract:
Ischemic Necrosis:
Reduced perfusion to the intestines can cause ischemic injury to the bowel, leading to bowel infarction and potentially fatal complications like perforation and peritonitis.
Liver:
Centrilobular Necrosis:
The liver’s ability to detoxify and synthesize proteins is compromised due to hypoperfusion, leading to liver failure.
Elevated liver enzymes and jaundice may develop.
Skin:
Pallor and Cold Extremities:
In shock, blood is diverted away from the skin to vital organs, causing cool, clammy skin and pallor.
Pathophysiology of Hemorrhage
Hemorrhage Overview:
Hemorrhage is the escape of blood from a ruptured vessel, leading to blood loss either externally or internally.
Types of Hemorrhage:
Petechiae:
Small (1-2 mm) pinpoint hemorrhages in the skin or mucous membranes, often due to platelet dysfunction or capillary fragility.
Purpura:
Slightly larger (3-5 mm) hemorrhages, commonly seen in conditions like vasculitis or coagulation disorders.
Ecchymoses (Bruises):
Larger hemorrhages (1-2 cm) that appear as skin discolorations due to the breakdown of hemoglobin from red blood cells.
Hemothorax, Hemopericardium, Hemoperitoneum, Hemarthrosis:
Large accumulations of blood in body cavities (e.g., chest, abdomen, joints) resulting from trauma or ruptured blood vessels.
Clinical Implications:
Significant hemorrhage can lead to hypovolemic shock, where the body cannot maintain adequate blood pressure and organ perfusion.
Chronic blood loss can result in iron-deficiency anemia as the body loses red blood cells and iron over time.