Interpreting and Reacting with Earth Systems (OCR)
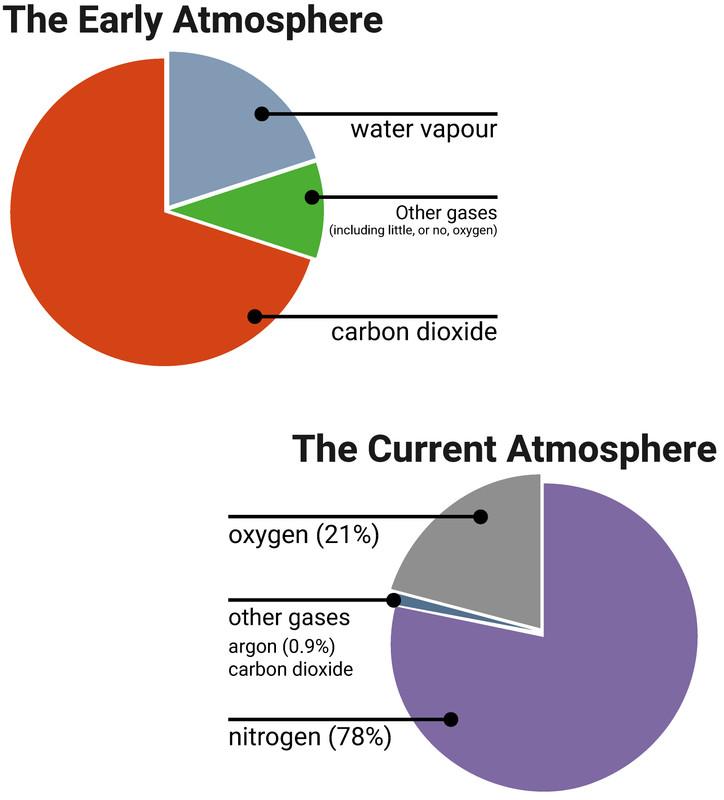
Early atmosphere
The early Earth's atmosphere is believed to have developed from gasses created by the planet's volcanic activity. It is believed that the early atmosphere included little to no oxygen, a lot of carbon dioxide, and tiny amounts of other gasses like ammonia and methane, much like the atmospheres of Mars and Venus today.
In addition, a great deal of water vapor was present in the atmosphere, which later condensed to create oceans as the Earth cooled.
The air's carbon dioxide content decreased over time. One method involved the gas dissolving and creating carbonates in the newly formed oceans. After that, they either precipitated into sediments or sea life formed calcium carbonate shells by using the dissolved carbon dioxide.
The development of algae and primitive plants, which required carbon dioxide for photosynthesis and subsequently created oxygen, which progressively increased the amount of oxygen in the atmosphere, was another factor in the decline in carbon dioxide levels.
carbon dioxide + water → glucose + oxygen
6CO2 + 6H2O → C6H12O6 + 6O2
Plants underwent evolution over a few billion years, and the percentage of oxygen rose to a point where animal evolution was possible. Because of how reactive oxygen can be, this almost put an end to all other life on the earth. We call this The Great Oxygenation Event.
Testing for oxygen
A luminous splint is used in the oxygen test, and it is placed within a gas container. There is oxygen present if the flame rekindles.
Combustion
Fuels react with the air's oxygen when they burn. Complete combustion will occur when there is an excess of air. This indicates that water and carbon dioxide are created.
Incomplete combustion occurs when there is insufficient oxygen in the air, producing carbon monoxide and water. We can argue that the carbon (as well as hydrogen) has been oxidized in both situations. These two kinds of combustion release energy since they are exothermic reactions.
Complete combustion
fuel + oxygen (abundant) → carbon dioxide + water
Incomplete combustion
fuel + oxygen (low levels) → carbon monoxide + water (+ carbon particulates)
Pollutants from Fuels
The primary cause of air pollution is the burning of fuels.
CO, or carbon monoxide
This gas is invisible and poisonous, binding to red blood cells' hemoglobin rather than oxygen. Because of this, there is less oxygen in the blood, which makes the affected individuals feel drowsy or even unconscious.
Severe poisoning can possibly be fatal.
Carbon particles, such as soot (C)
This may obstruct the pipes that remove waste gasses from an appliance. Buildings will turn black from it, and breathing issues may arise if it gets into the lungs.
Particulates can also be a factor in global dimming, which lowers the quantity of sunlight that reaches the surface of the Earth.
Sulfur dioxide (SO2)
When fossil fuels, such as coal and crude oil, are burned, sulfur impurities in the fuels can combine with oxygen to form sulfur dioxide gas.
Acid rain, which ruins buildings and wildlife habitats including lakes and forests, is created when this gas dissolves in rains.
Nitrogen oxide (NOx)
Nitrogen from the air and oxygen from burning fuels in engines can react at high temperatures to form nitrogen oxides. Acid rain can also be created by dissolving these in rainfall.
Reddish-brown in color, nitrogen dioxide is a hazardous gas that can lead to respiratory conditions including bronchitis. Most NOx gasses in automobiles are converted back to safe nitrogen by catalytic converters.
Certain nitrogen oxides combine with other contaminants to create dangerous "photochemical smog."
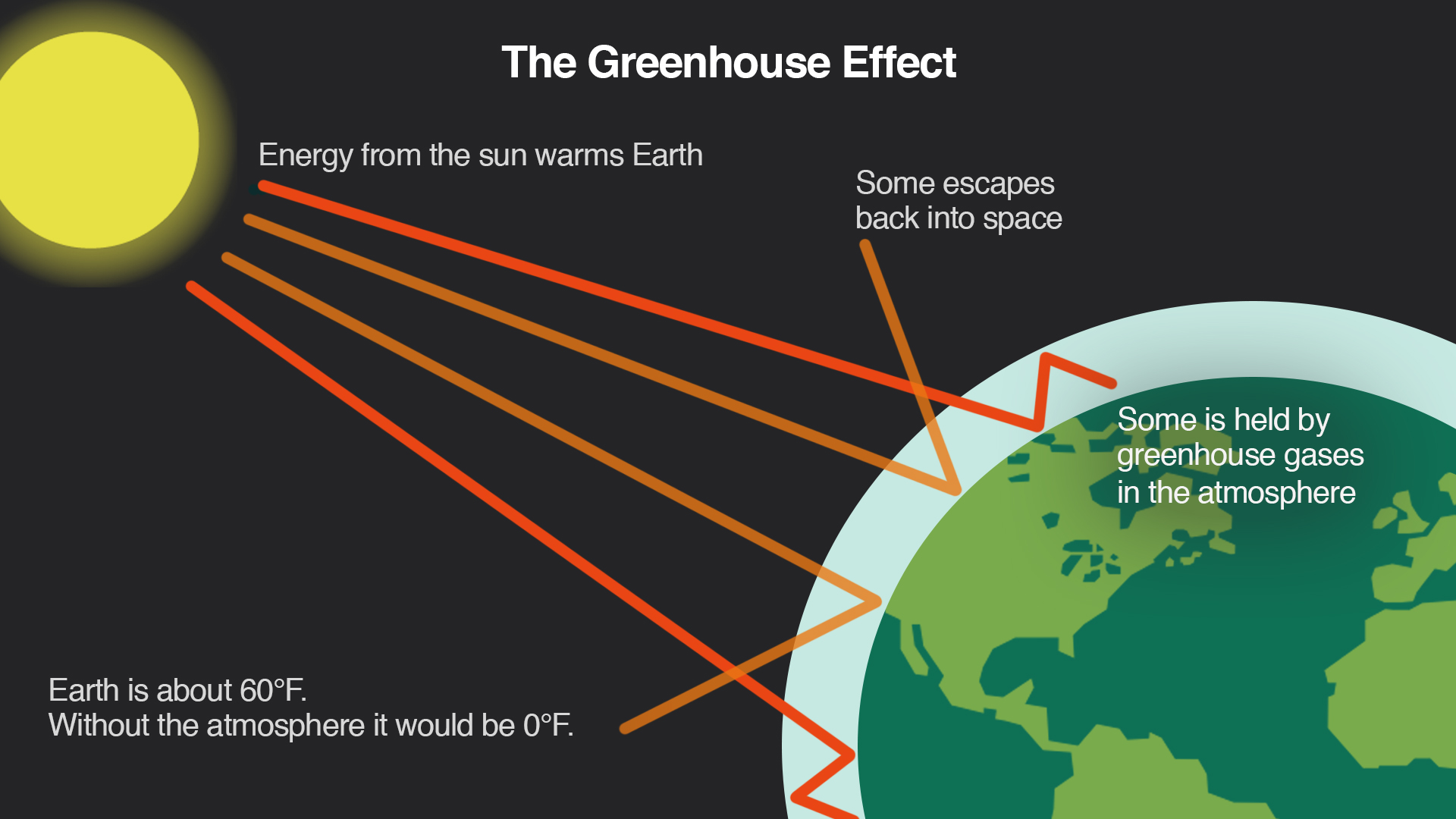
The Greenhouse Effect
The Earth's atmosphere contains greenhouse gasses that keep the planet's temperature high enough to support life. Methane, carbon dioxide, and water vapor are examples of greenhouse gasses.
The greenhouse effect is the result of them absorbing heat emitted by the Earth and then releasing energy to keep it warm.
The Sun's energy is transmitted to Earth by waves, including infrared radiation and light.
The Earth's surface absorbs some energy and becomes warmer as a result.
Earth's heat causes it to release infrared radiation.
Certain gasses in the atmosphere take in the energy carried by these infrared waves, release it again, and some of it returns to warm the Earth's surface
Greenhouse gasses:
carbon dioxide (CO2)
methane (CH4)
water (H2O)
Climate Change's Anthropogenic (Human Activity) Causes
Many scientists think that human activity (producing large amounts of greenhouse gasses) will raise the temperature of the Earth's atmosphere, leading to global climate change, based on peer-reviewed research.
Because climate change is a complicated topic, modeling this is challenging. This results in oversimplified models, conjecture, and media-expressed judgments that can be skewed and based on incomplete or partial evidence.
Fossil fuels have been burned more often for industry and energy since roughly 1850. Since carbon dioxide is released when fossil fuels are burned, the fact that global carbon dioxide levels have been rising during this time is strong proof that the two are connected.
The average temperature of the Earth's surface has increased along with the concentrations of carbon dioxide. Although there is a high correlation between the two, this does not imply that there is a causative relationship, where one causes the other.
In the lab, scientists have demonstrated that carbon dioxide absorbs infrared radiation. Satellite data has also demonstrated that as carbon dioxide concentrations have risen, less infrared light has been able to escape the Earth's atmosphere.
Assessing the available evidence
Measuring the quantities of gasses trapped in ice cores—the oldest of which date back 800,000 years—provides evidence for ancient carbon dioxide levels.
Since they only come from one location, the oldest temperature records for central England, which go back to 1659, cannot be used to evaluate variations in world temperatures. Global temperature records date back to only approximately 1880. Furthermore, these measurements lacked precision.
Climate Change
Because methane is stronger at collecting infrared radiation than carbon dioxide, it is a far more potent greenhouse gas—some experts claim up to four times more—than carbon dioxide.
When natural gas and oil are collected from the ground and processed, methane—the primary component of natural gas—is released into the atmosphere.
The bacteria in livestock's stomachs during digestion also causes a large amount of methane gas to be produced, especially in cattle. Rice fields and garbage sites both contain comparable microorganisms.
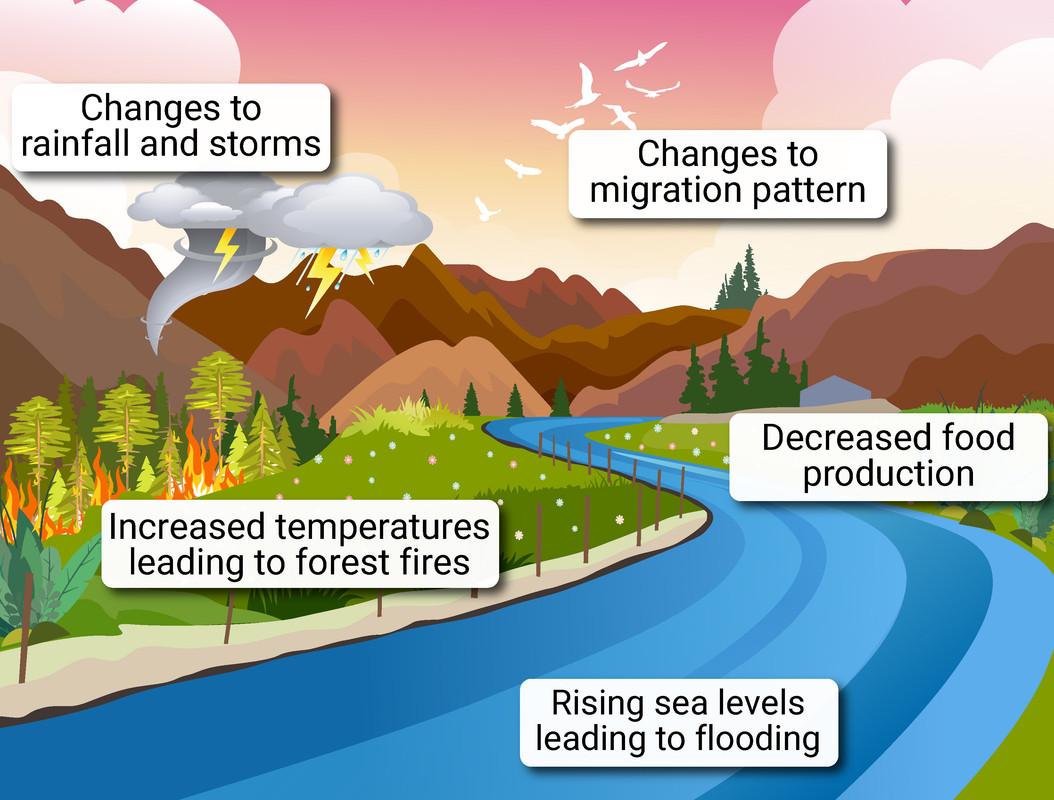
Effects of Climate Change
The following effects of rising temperatures brought on by an increase in carbon dioxide and methane in the atmosphere:
global sea levels will rise as a result of the melting of ice in glaciers and at the South Pole, which will increase and maybe permanently flood some areas.
animals will seek out cooler places to live after leaving their "natural" habitats, and certain plants and animals might go extinct if they are unable to withstand the rising temperatures.
weather patterns will start to shift, with some regions getting significantly drier and others much wetter. Severe storms and heat waves/droughts will also become more common.
crop growth and wildlife will be impacted by the constantly shifting weather patterns.
more carbon dioxide released into the atmosphere will dissolve and reduce the pH of water systems, endangering the life forms that inhabit them.
Reducing the Effects
While using renewable energy sources can help reduce greenhouse gas emissions, it might not be able to reverse the harm already done. Some have proposed returning infrared radiation back into space or collecting carbon dioxide and burying it.
Reducing the damage entails constructing irrigation systems, dams, and flood defenses; this is likely only a temporary solution.
These will most likely ruin habitats and just treat the symptoms of a much larger problem—they won't address the root of the problem.
Potable Water
Water is a necessity for all life. They require water that is of sufficient quality, which for humans implies that it must have minimal concentrations of bacteria and dissolved salts.
Potable water is defined as water that is safe to consume. Because it has dissolved materials in it, potable water is not pure water.
There are numerous ways to create drinkable water, and the one chosen will rely on the water resources that are accessible and the environmental factors in the area.
Rainfall in the United Kingdom (UK) creates fresh water, which is a mixture of dissolved elements that collects in lakes, rivers, and the ground. These steps must be completed for this water to become potable:
sedimentation: solids are eliminated when they settle to the bottom.
filtration: the removal of small particles like sand
chlorination: the use of chlorine to destroy microorganisms
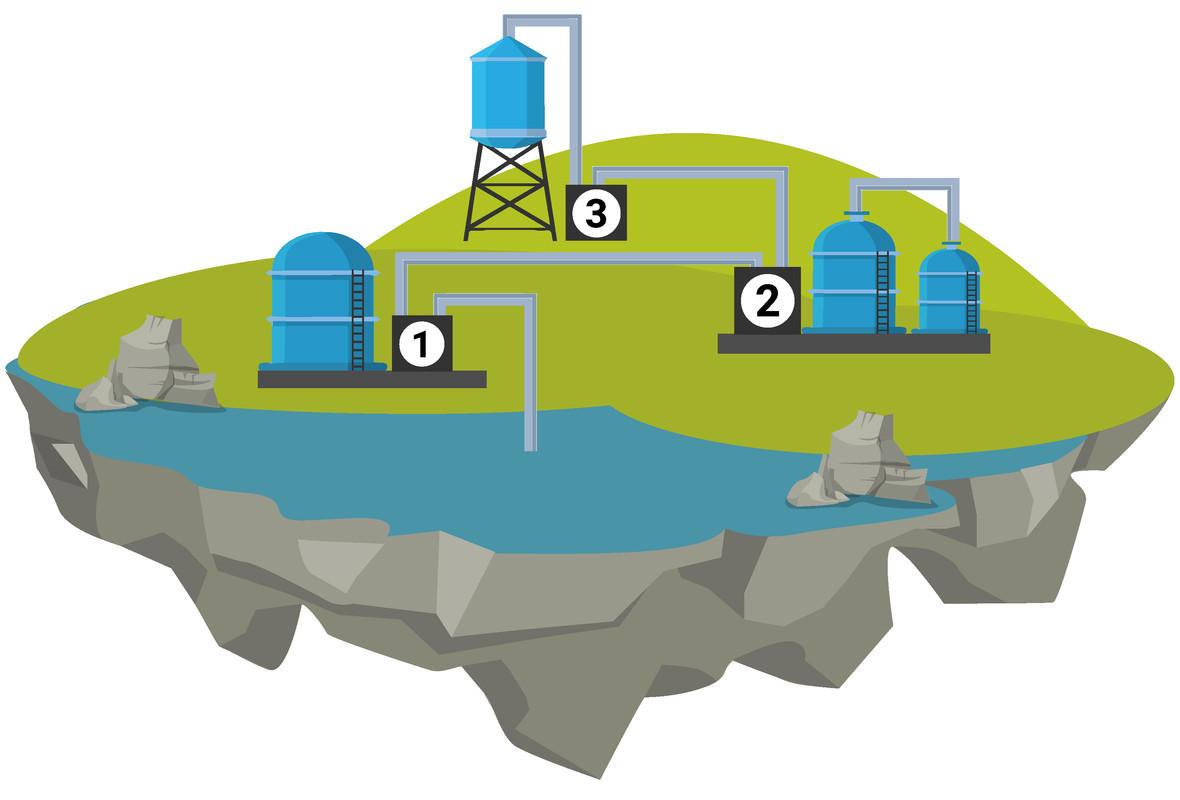
Sea water (high salinity) may need to be desalinated if freshwater resources are scarce (like on a small island). Distillation is one method of desalination. This procedure is expensive to maintain and uses a lot of energy.
In the laboratory, distilled water, also known as deionized water, is typically used for analysis. This is because the dissolved salts in tap water can affect our readings; therefore, we need to utilize pure water, which has had these salts eliminated.