Enzymes
Induced Fit Model
Active site is not initially complementary to the substrate
Molds itself around substrate - becomes more complementary on collision with substrate
Change of shape puts stress on the bonds of the substrate
Lowers Activation Energy required for reaction
Enzyme Reaction Rates
Enzyme Concentration
As concentration of enzyme increases, rate of reaction increases. This is because more enzymes, means a higher collision frequency between enzyme and substrate to create an enzyme substrate complex. (assuming plenty of substrate present)
Substrate Concentration
As concentration of substrate increases, rate of reaction increases until a point. This is because as more and more substrate collide with enzyme to create enzyme substrate complexes, there are less enzymes to be collided with, so rate of reaction slows down.
Temperature
The optimum temperature of an enzyme is the temperature in which the enzyme activity is at its highest.
Enzymes perform poorly at lower temperatures as there is not enough KE to collide with the substrate at E > Ea, and therefore unable to create a enzyme-substrate complex, lowering rate of reaction; too few collisions.
pH
Enzymes are denatured by pH. Deviations from this optimal range can interfere with the formation of the enzyme-substrate complex, reducing the rate of reaction. At extreme pH levels, enzymes can lose their catalytic activity due to irreversible changes in their shape, which affects their active site and inhibits substrate binding.
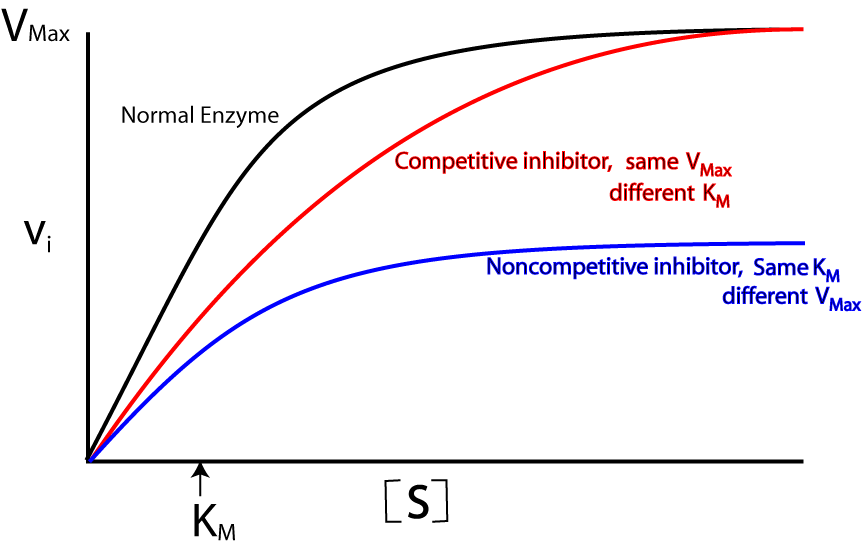
Enzyme Inhibitors
Prevent the substrate binding to the active site
Competitive Inhibitors
Reduces rate of reaction as they compete for the same active site, as it has a similar shape to the substrate.
It is possible to reduce the effect of the inhibitor by adding more substrate.
Non-Competitive Inhibitors
Changes shape of active site; Attaches to allosteric site on the enzyme.
This means the substrate is unable to enter the active site, and a reaction cannot occur, leaving the MAX to be lowered than the uninhibited Rate of Reaction
Enzyme Cofactors
Role of Cofactors
Completes the enzyme; needed for the enzyme to work
Enabling an Enzyme’s Catalytic Ability
The cofactor binds with the apoenzyme to create the active site, and therefore start catalytic activity or make active site more reactive.
Apoenzyme and a Cofactor
The apoenzyme is a protein, and the cofactor is a non-protein → together they create a functional enzyme.
Types of Cofactors
Prosthetic Group
Permanently attached to the apoenzyme
Coenzyme
Becomes detached after the reaction and may take place in other reactions
The Active Site
An enzyme has an active site and it is specific and complementary to the shape of the substrate