2.1.2 Chemical tests
2.1.2 Biological molecules (q-s)
Chemical tests and chromatography - Notes
Chemical tests
2.1.2q: how to carry out and interpret the results of the following chemical tests: biuret test for proteins, Benedict’s test for reducing and non-reducing sugars, reagent test strips for reducing sugars, iodine test for starch, emulsion test for lipids.
- Draw a table that shows the chemicals used, an outline of the method, the appearance of a negative results and the appearance of a positive result for the biuret test for proteins, the Benedict’s test for reducing sugars, the Benedict’s test for non-reducing sugars, the iodine test for starch and the emulsion test for lipids. (F)
- Define the terms “qualitative test”, “semi-quantitative test” and “quantitative test”. (F)
- Explain how the Benedict’s test for reducing sugars can act as a semi-quantitative test (include the colour range that can be seen with different concentrations of glucose).
- List 5 examples of reducing sugars.
- State one example of a non-reducing sugar.
- Explain why reducing sugars are called “reducing” sugars. (S+C)
2.1.2r: quantitative methods to determine the concentration of a chemical substance in solution.
- Describe, in principle, how a colorimeter works. (F)
- Define the terms “percentage absorbance” and “percentage transmission” in relation to data provided by a colorimeter.
- Explain how to convert percentage transmission into percentage absorbance.
- Explain how a colorimeter can be used in a quantitative test for reducing sugars.
- Define the term “calibration curve” and explain how they are used to identify the concentration of glucose in a solution.
Chromatography
2.1.2s: (i) the principles and uses of paper and thin layer chromatography to separate biological molecules / compounds (ii) practical investigations to analyse biological solutions using paper or thin layer chromatography.
- Describe the purpose of chromatography. (F)
- Describe a step by step method for conducting chromatography to identify the components of an unknown mixture of dissolved substances (e.g. proteins, carbohydrates, amino acids vitamins or nucleic acids). For each step explain why it is necessary and why it is done the way it is. (F)
- Draw a diagram of a chromatogram showing how to calculate the Rf values of each spot. (F)
- Explain how to use Rf values to identify the molecules present in a solution. (F)
- Explain what determines how far a particular molecule travels in chromatography.
- Describe 3 different ways to do chromatography. (S+C)
- Describe what “two-way chromatography” is and explain why it is useful. (S+C)
Reviewing chemical tests
Molecule being tested for | Name of test | Method | Colour change of positive test |
Reducing sugar (e.g. Glu, Gal, Fru, Mal, Lac) | Benedict’s test for reducing sugars |
| Blue to brick red |
Non-reducing sugar (e.g. Suc) | Benedict’s test for non-reducing sugars |
| Negative result for initial reducing sugar test then Blue to brick red |
Starch | Iodine test |
| Orange-brown to blue-black |
Lipid | Emulsion test |
| Colourless to cloudy white emulsion |
Protein | Biuret test |
| Pale blue to purple |
Terminology
Qualitative test
- Tests for the presence or absence of a substance (e.g. Benedict’s test PAG, Biuret test PAG, Emulsion test, Iodine test for starch)
Semi-Quantitative test
- Identifies the relative concentration of a substance in solution i.e. higher or lower than other solutions but not an exact value. So long as there is also data from known concentrations, the concentration of an unknown solution can be identified as within a particular range.
Quantitative test
- Identifies a value for the concentration of a substance in solution. This is a numerical value. It requires a calibration curve to have been drawn using data from solutions of known concentration.
Subjective
- Data collected by eye. It is based on a decision made by the experimenter. It could vary from experimenter to experimenter (e.g. all the chemical test we have done so far but particularly the semi-quantitative glucose test).
Objective
- Numerical data provided by a piece of equipment. No decision on the value is made by the experimenter so every experimenter would record that value from that piece of data collection.
Colorimeters
A colorimeter detects how much light is transmitted through a coloured liquid. It provides an objective, quantitative measurement of the colour of a liquid.
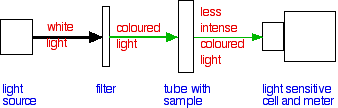
To use a colorimeter first the correct filter needs to be selected. The colour of light shining through the sample needs to be a wavelength of light (i.e. colour) that the sample will absorb. For example, if the sample is blue, an orange filter tends to be used. Whereas if the sample is purple then a green filter tends to be used.
The colourimeter detects how much light has passed through the sample and it compares this to how much light passes through a tube with just water in it. The display can be set to read percentage transmission (where water = 100% transmission and no light getting through is 0% transmission) or absorbance. Percentage absorbance is just 100 minus the transmission value but absorbance is also often presented on an arbitrary scale from 0 to 2.
Colourimeters can be used to do quantitative testing by sampling known concentrations of a liquid and plotting a calibration curve (see an example below).
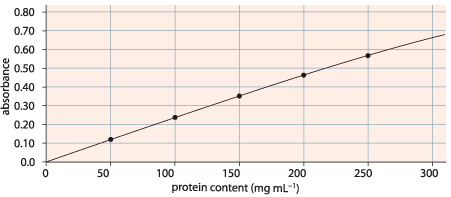
Once the calibration curve has been made unknown concentrations can be tested in the colorimeter. The value the colorimeter gives (absorbance in the example above) is then found on the y-axis, a horizontal line is drawn across from that value to the calibration curve, a vertical line is drawn down to the x-axis from the place where the horizontal line hits the calibration curve. The concentration of the unknown sample is then determined by the concentration where the vertical line hits the x-axis.
Chromatography
Chromatography separates molecules in a liquid mixture and allows each molecule to be identified. It is a qualitative test.
- Draw a pencil line on the chromatography plate about 1cm from the bottom edge [pencil marks don’t move during the process] [this is to mark the origin to allow measurements to be taken later]. Only handle the plate at the edges [to avoid damaging the plate and to avoid contaminating the plate with chemicals from hands].
- Spot the sample solution onto the pencil line using a capillary tube. Allow the spot to dry and the spot again in the same place. Repeat this process several times [this creates a small concentrated spot of the sample].
- Place the plate in a jar containing solvent. Ensure that the plate doesn’t touch the sides of the jar (except at the very top) [so that the solvent will run directly up the plate and not move in different directions due to interactions with the side of the jar]. The solvent is no more than 0.5cm deep in the jar [so that the spot doesn’t dissolve in the solvent at the bottom of the jar].
- Put a lid on the jar [this avoids too much solvent evaporating].
- Let the chromatogram run until the solvent is about 1cm from the top of the plate [if the solvent reaches the top of the plate it is not possible to make accurate measurements].
- Remove the chromatogram from the jar and immediately draw a pencil line along the solvent front [so that when the plate dries it is still possible to see how far up the plate the solvent reached]. Allow the plate to dry.
- If the molecules from the sample are not coloured then the plate needs to be treated with a dye to determine where the chemicals in the sample ended up on the plate (e.g. Ninhydrin is used to turn amino acids from colourless to a purple/brown colour).
- Once the spots of chemicals are observable the Rf (retention factor) value of each spot needs to be calculated.

- The measured Rf values of each spot can be compared to known Rf values for different chemicals to identify the chemical in each spot.
Chromatography uses a solvent (the mobile phase) and a solid that the solvent moves up through (the stationary phase).
In ‘thin layer chromatography’ the stationary phase is silica gel and the mobile phase is an organic solvent (it’s actually a mixture of a variety of organic solvents).
Different chemicals have different Rf values because they vary in how soluble they are in the solvent and the affinity they have for the stationary phase.