Chapter 16: Solids, Liquids, and Gases
Section 1: Kinetic Theory
States of Matter
- Kinetic Theory: an explanation of how particles in matter behave.
- The three assumptions of the kinetic theory are as follows:
- All matter is composed of small particles (atoms, molecules, and ions).
- These particles are in constant, random motion.
- These particles are colliding with each other and the walls of their container.
- Particles lose some energy during collisions with other particles.
- Atoms in solids are held tightly in place by the attraction between the particles.
- Thermal energy is the total energy of a material’s particles, including kinetic—vibrations and movement within and between the particles—and potential—resulting from forces that act within or between particles.
- In science, temperature means the average kinetic energy of particles in the substance, or how fast the particles are moving.
- Molecules will have kinetic energy at all temperatures, including absolute zero.
- The particles in a solid are packed together tightly and are constantly vibrating in place.
- Most solid materials have a specific type of geometric arrangement in which they form when cooled.
- Chemical and physical properties of solids often can be attributed to the type of geometric arrangement that the solid forms.
- The particles in solid water align themselves in an ordered geometric pattern. Even though a solid ice cube doesn’t look like it is moving, its molecules are vibrating in place.
- Melting Point: the temperature at which a solid begins to liquefy.
- Heat of Fusion: The amount of energy required to change a substance from the solid phase to the liquid phase at its melting point
- The particles in a liquid are moving more freely than the particles in a solid. They have enough kinetic energy to slip out of the ordered arrangement of a solid.
- The particles in a liquid have not completely overcome the attractive forces between them. This causes the particles to cling together, giving liquids a definite volume.
- In gases, the particles are far apart and the attractive forces between the particles are overcome. Gases do not have a definite volume or shape.
- Gas particles have enough kinetic energy to overcome the attractions between them.
- The particles that are moving fast enough can escape the attractive forces of other particles and enter the gas state. This process is called vaporization.
- Vaporization can occur in two ways— evaporation and boiling.
- Evaporation is vaporization that occurs at the surface of a liquid and can occur at temperatures below the liquid’s boiling point.
- Boiling occurs throughout a liquid at a specific temperature depending on the pressure on the surface of the liquid.
- Boiling Point: the temperature at which the pressure of the vapor in the liquid is equal to the external pressure acting on the surface of the liquid.
- Heat of Vaporization: the amount of energy required for the liquid at its boiling point to become a gas.
- The movement of particles and the collisions between gas particles cause gases to diffuse.
- Diffusion: the spreading of particles throughout a given volume until they are uniformly distributed.
- Diffusion occurs in solids and liquids but occurs most rapidly in gases.
- After the attractive forces are overcome, particles move more freely and their average kinetic energy, or temperature, increases.
- Scientists estimate that much of the matter in the universe is plasma.
- Plasma: matter consisting of positively and negatively charged particles.
- Although this matter contains positive and negative particles, its overall charge is neutral because equal numbers of both charges are present.
- Stars including the Sun contain matter that is in the plasma phase. Plasma exists where the temperature is extremely high.
Thermal Expansion: an increase in the size of a substance when the temperature is increased.
- The movements of the particles closer together result in an overall shrinking of the object, known as contraction.
- Expansion and contraction occur in most solids, liquids, and gases.
- Water molecules are unusual in that they have highly positive and highly negative areas.
- The positively and negatively charged regions on a water molecule interact to create empty spaces in the crystal lattice. These interactions cause water to expand when it is in the solid phase.
- As the temperature of water drops, the particles move closer together.
- Solid ice is less dense than liquid water.
Solid or a Liquid?
- Amorphous solids and liquid crystals are two classes of materials that do not react as you would expect when they are changing states.
- Not all solids have a definite temperature at which they change from solid to liquid.
- The particles that make up amorphous solids are typically long, chainlike structures that can get jumbled and twisted instead of being neatly stacked into geometric arrangements.
- Liquids do not have an orderly arrangement of particles.
- Liquid crystals are another group of materials that do not change states in the usual manner.
- Liquid crystals are placed in classes depending upon the type of order they maintain when they liquefy.
Section 2: Properties of Fluids
- How do ships float?
- Buoyancy: the ability of a fluid—a liquid or a gas—to exert an upward force on an object immersed in it.
- If the buoyant force is equal to the object’s weight, the object will float.
- An object will float if its density is less than the density of the fluid it is placed in.
- Pascal’s Principle
- Pressure: force exerted per unit area, or P=F/A.
- According to Pascal’s principle, pressure applied to a fluid is transmitted throughout the fluid.
- Hydraulic machines are machines that move heavy loads in accordance with Pascal’s principle.
Question: A barber raises his customer’s chair by applying a force of 150N to a hydraulic piston of area 0.01 m2. If the chair is attached to a piston of area 0.1 m2, how massive a customer can the chair raise? Assume the chair itself has a mass of 5 kg.
Answer: To solve this problem, first determine the force applied to the larger piston.
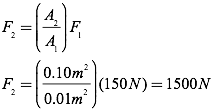
If the maximum force on the chair is 1500N, you can now determine the maximum mass which can be lifted by recognizing that the force that must be overcome to lift the customer is the force of gravity, therefore the applied force on the customer must equal the force of gravity on the customer.
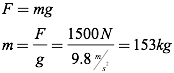
If the chair has a mass of 5 kilograms, the maximum mass of a customer in the chair must be 148 kg.
- Bernoulli’s Principle
- According to Bernoulli’s principle, as the velocity of a fluid increases, the pressure exerted by the fluid decreases.
- Bernoulli’s principle was used in designing the hose end sprayer
- Fluid Flow
- Another property exhibited by fluid is its tendency to flow.
- Viscosity: The resistance to flow by a fluid
- When a container of liquid is tilted to allow flow to begin, the flowing particles will transfer energy to the particles that are stationary.
- If the flowing particles do not effectively pull the other particles into motion, then the liquid has a high viscosity, or a high resistance to flow. If the flowing particles pull the other particles into motion easily, then the liquid has low viscosity, or a low resistance to flow.
Section 3: Behavior of Gases
- Pressure
- Pressure is the amount of force exerted per unit of area.
- Pascal: the SI unit of pressure.
- Boyle’s Law
- If you squeeze gas into a smaller space, its particles will strike the walls more often—giving an increased pressure.
- If you give the gas particles more space, they will hit the walls less often—gas pressure will be reduced.
- According to Boyle’s law, if you decrease the volume of a container of gas and hold the temperature constant, the pressure of the gas will increase.
- As pressure is decreased the volume increases. As the pressure is increased, the volume will decrease.
- When Boyle’s law is applied to a real life situation, we find that the pressure multiplied by the volume is always equal to a constant if the temperature is constant.
- The Pressure-Temperature Relationship
- If the pressure becomes greater than the canister can hold, it will explode. At a constant volume, an increase in temperature results in an increase in pressure.
- Charles’s Law
- According to Charles’s law, the volume of a gas increases with increasing temperature, as long as pressure does not change.
- The volume of a gas shrinks with decreasing temperature
- Charles’s law can be explained using the kinetic theory of matter.
- As a gas is heated, its particles move faster and faster and its temperature increases.
- When using Charles’s law, the pressure must be kept constant.