6.Physical Properties
Alkyl Halides Properties and Boiling Points
Pure Alkyl Halides
Colorless in pure form
Bromides and iodides develop color when exposed to light
Many volatile halogen compounds have a sweet smell
Physical State at Room Temperature
Methyl chloride, methyl bromide, ethyl chloride, and some chlorofluoromethanes are gases
Higher members are liquids or solids
Molecular Polarity and Intermolecular Forces
Organic halogen compounds are generally polar
Stronger intermolecular forces due to greater polarity and higher molecular mass compared to hydrocarbons
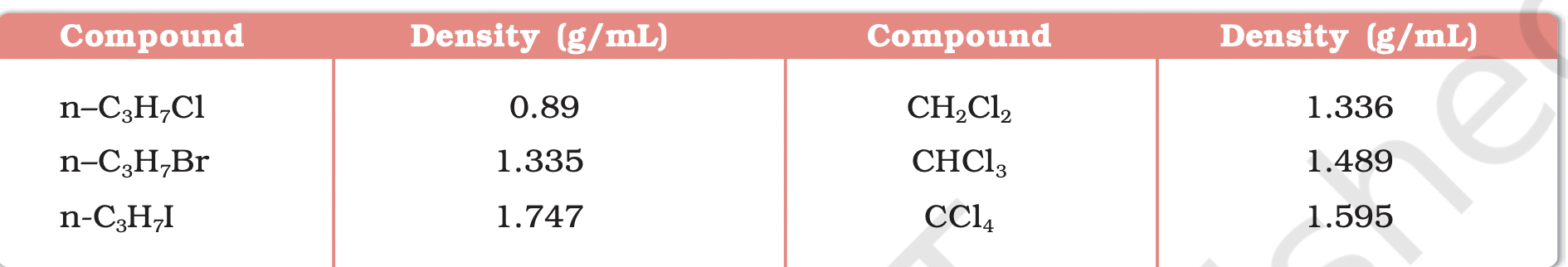
Boiling points of halogen derivatives (chlorides, bromides, iodides) are higher than hydrocarbons of similar molecular mass
Effect of Halogen Size on Boiling Points
Boiling points decrease in order: RI > RBr > RCl > RF
Increase in size and mass of halogen atom leads to stronger van der Waals forces
Attractions strengthen with larger molecules and more electrons
Boiling Points of Isomeric Haloalkanes and Branching
Introduction
Explanation of isomeric haloalkanes
Relationship between boiling point and branching in haloalkanes
Boiling Points of Isomeric Haloalkanes
Definition of boiling point
Influence of molecular structure on boiling point
Effect of Branching on Boiling Points
Explanation of branching in organic compounds
Impact of branching on intermolecular forces
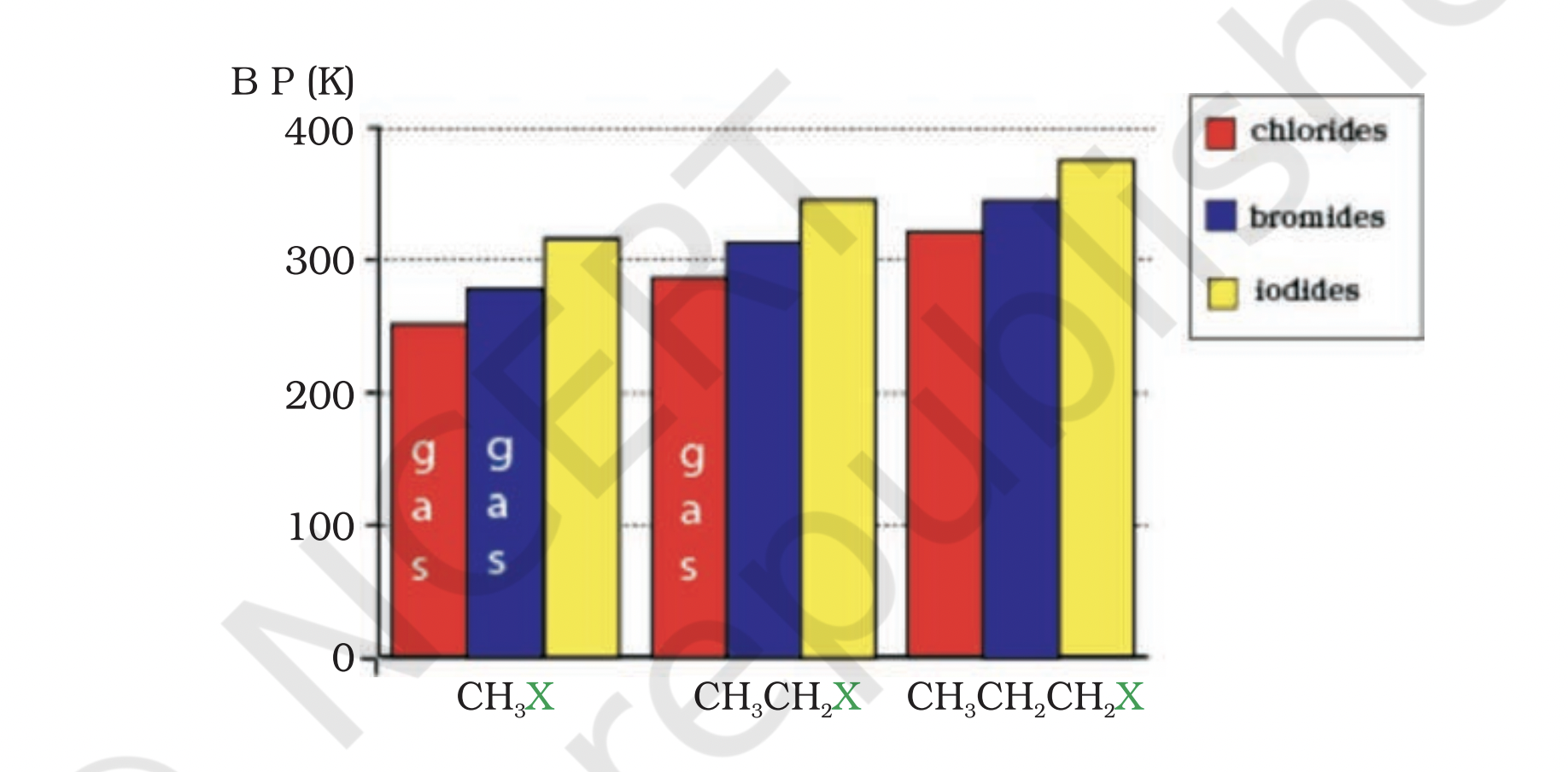
Comparison of Isomeric Haloalkanes
Comparison of 1-bromo-2-methylpropane, 2-bromo-2-methylpropane, and 2-bromopropane
Explanation of the lowest boiling point in 2-bromo-2-methylpropane
Conclusion
Recap of the relationship between branching and boiling points in isomeric haloalkanes
Importance of understanding structure-property relationships in organic compounds
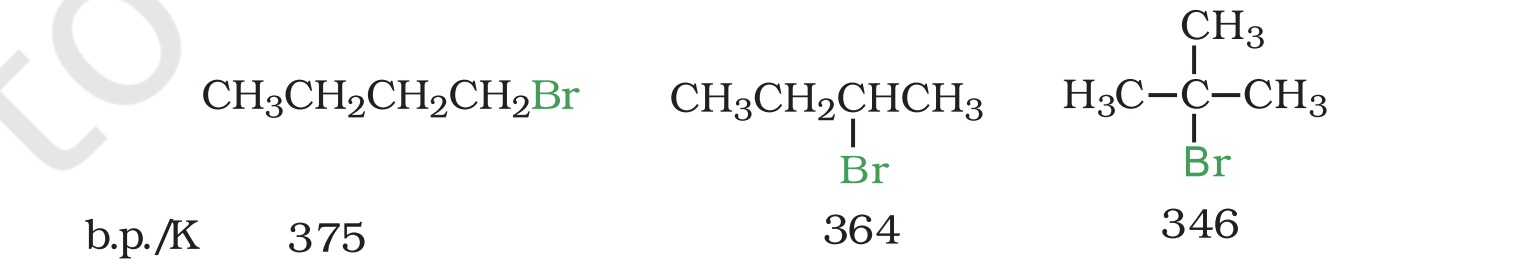
Boiling Points and Melting Points of Isomeric Dihalobenzenes
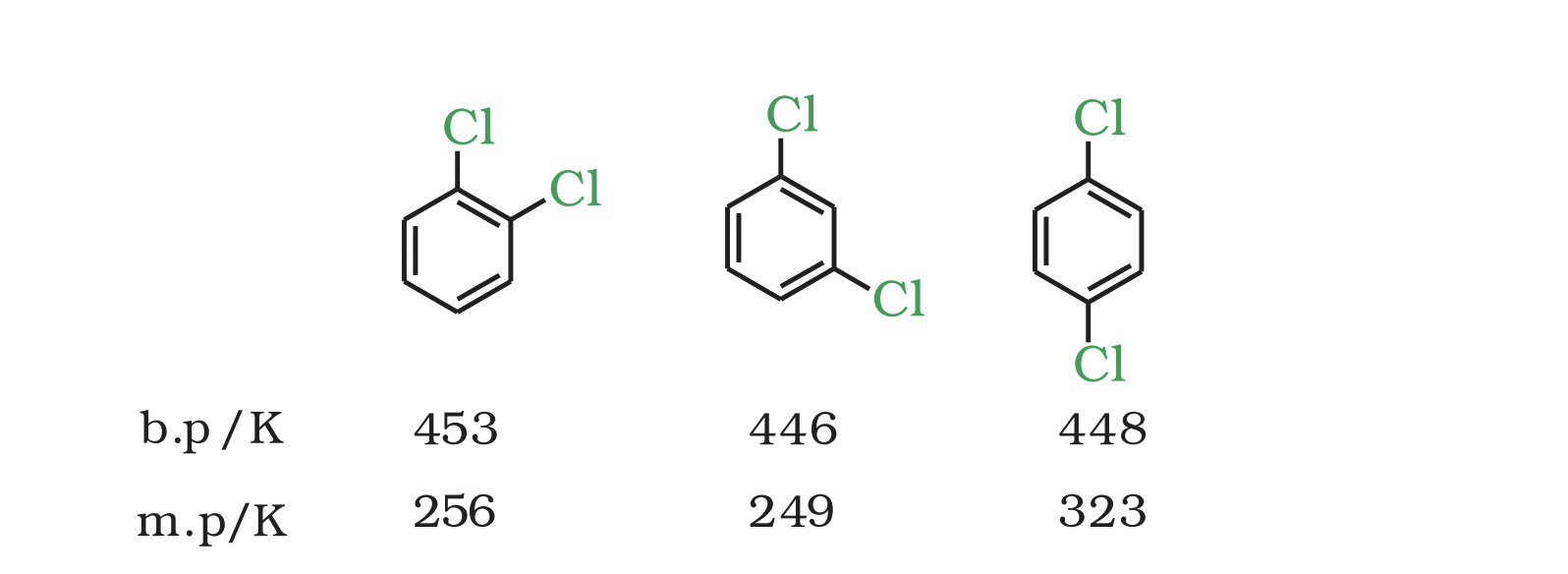
Boiling Points of Isomeric Dihalobenzenes
Boiling points of isomeric dihalobenzenes are very similar.
Para-isomers have higher melting points compared to ortho and meta-isomers.
Reason for Higher Melting Points of Para-Isomers
Symmetry of para-isomers allows them to fit better in crystal lattice.
Better fit in crystal lattice leads to higher melting points.
Density of Halogen Derivatives
Bromo, iodo, and polychloro derivatives of hydrocarbons are denser than water.
Factors Affecting Density
Density increases with:
Increase in the number of carbon atoms.
Increase in the number of halogen atoms.
Increase in the atomic mass of the halogen atom.
Solubility of Haloalkanes
Solubility in Water
Very slightly soluble in water
Energy required to dissolve haloalkane in water
Overcoming attractions between haloalkane molecules and breaking hydrogen bonds in water
New attractions between haloalkane and water molecules are weaker than original hydrogen bonds in water
Low solubility in water due to weaker attractions
Solubility in Organic Solvents
Haloalkanes tend to dissolve in organic solvents
New intermolecular attractions between haloalkanes and solvent molecules
Strength of attractions between haloalkanes and solvent molecules similar to those being broken in separate molecules