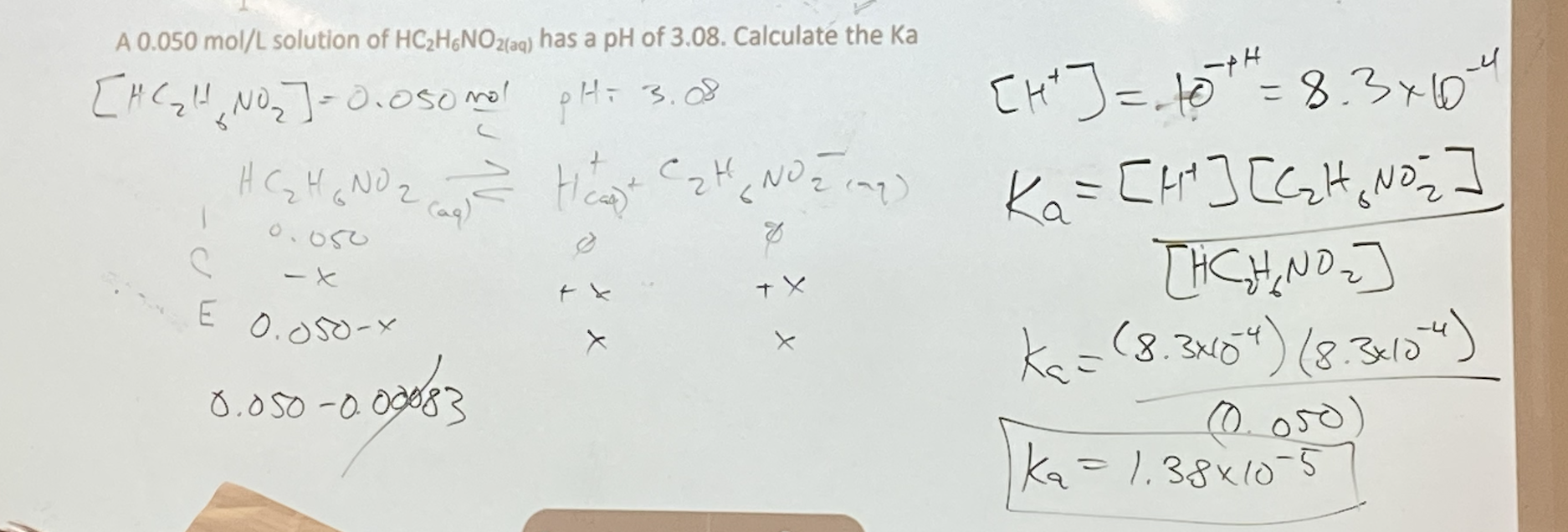8.4 Calculations Involving Acidic Solutions
Strong Acids
Take a bottle of 1.0 mol/L HCL(aq). It is a strong acid. What entities are in that bottle?
- H+
- Cl-
- H2O (it auto-ionizes: can turn into hydronium or hydroxide, hydronium doesn’t matter that much with a strong acid)
To calculate the pH:
- In strong acids: we assume 100% ionization. In a solution of 0.25 mol/L of nitric acid, calculate the pH and pOH.
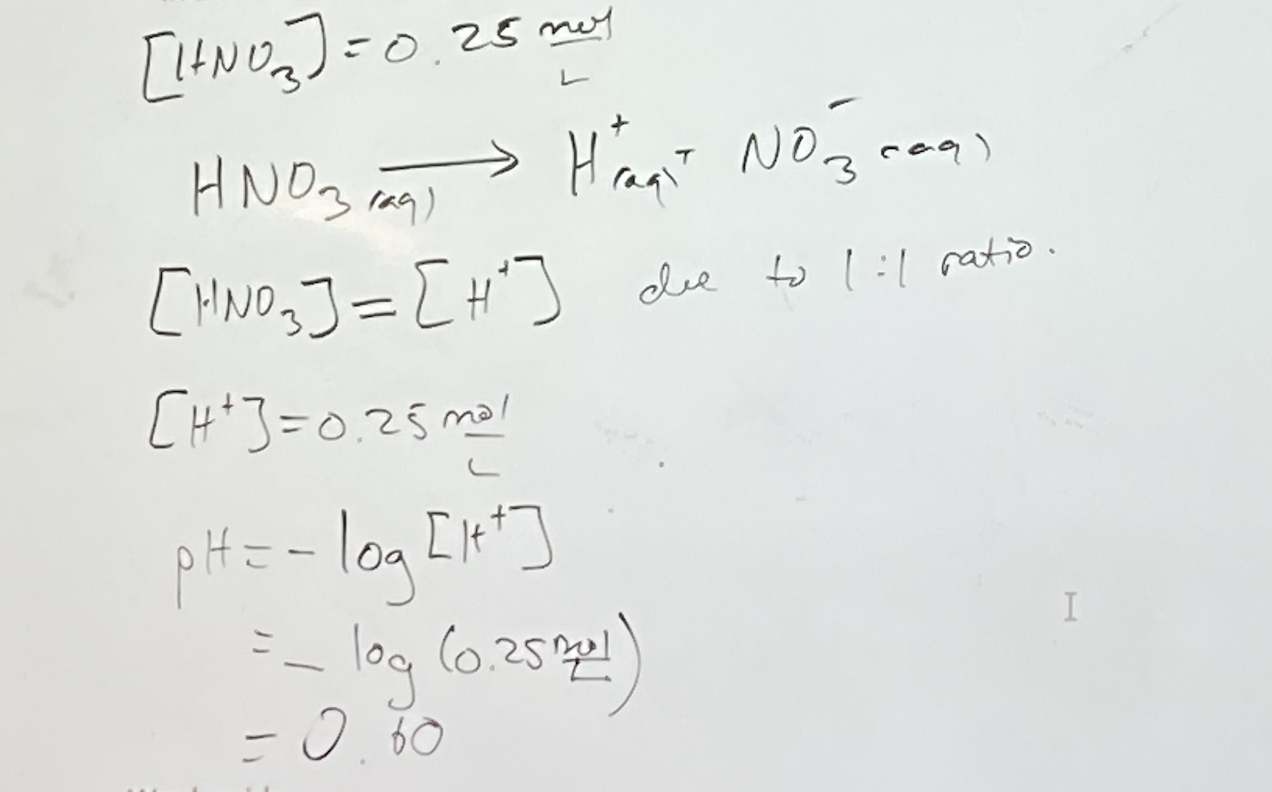
Weak Acids
- Find the percentage ionization (% of solute that ionizes when dissolved in solvent).
- % ionization = [H+] / [HA] x 100
Calculations Involving Acidic Solutions
A solution of 0.10 mol/L methanoic acid has a pH of 2.38. What is the % ionization?
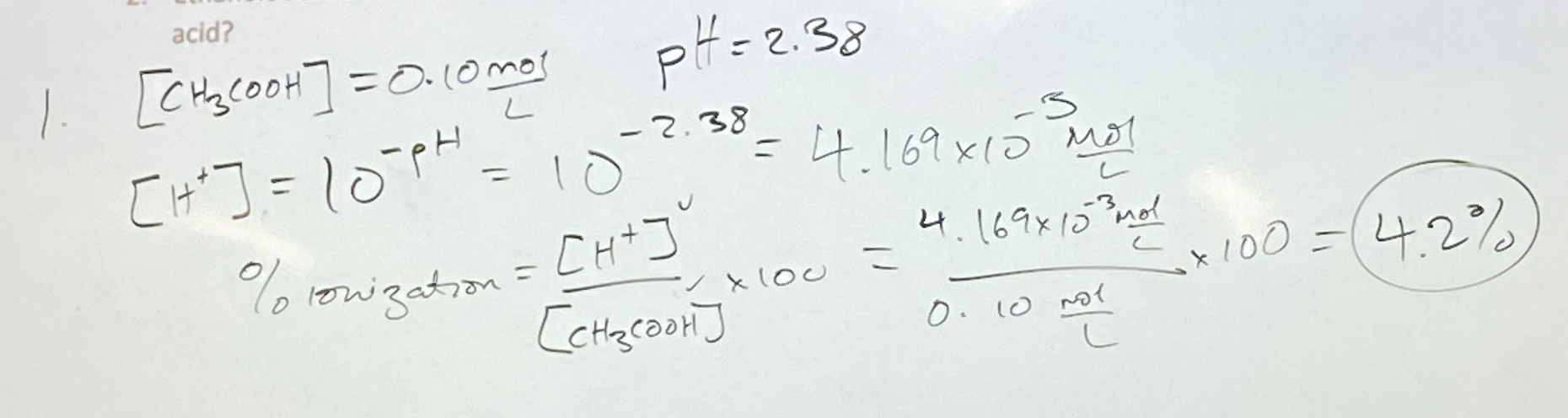
Ethanoic acid has a % ionization of 1.3%. What is the Ka of a 0.1000 mol/L solution of ethnoic acid?

Calculating pH of acidic solutions: (similar to equilibrium problems)
Identify major entities in the solution at equilibrium
Decide which of the major entities produces H+ ions
Identify major sources of H+ ions
Write the equilibrium constant equation for the reaction (primary source of H+)
Use ICE to determine changes at equilibrium, solve for x (don’t forget the 100 / 5% rule!)
Calculate the [H+]eq
Calculate pH
- Ex: What is the pH of 0.100 mol/L hypochlorous acid (See appendix B5 for Ka value)
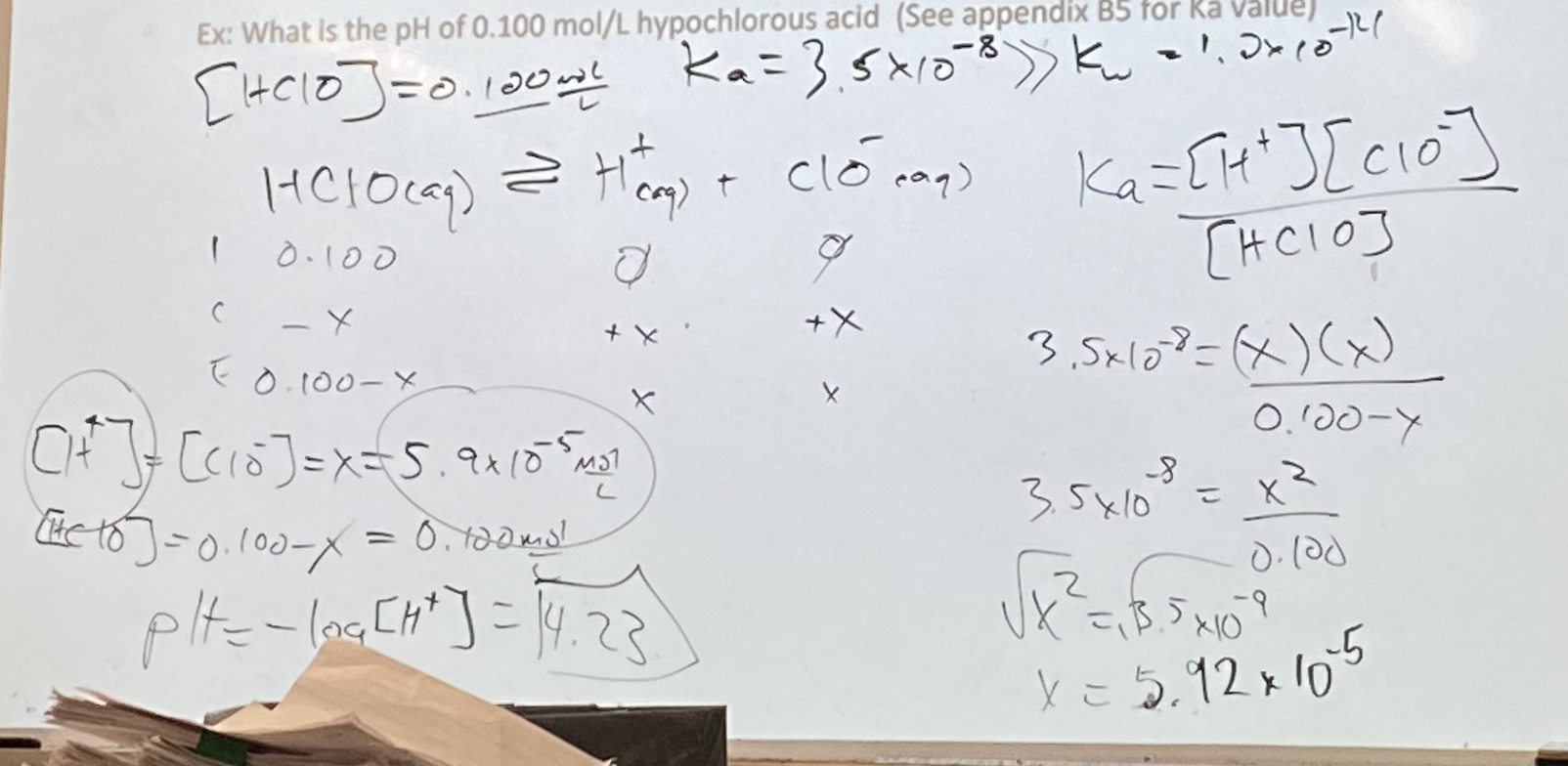
- Calculating Ka of a weak acid:
Identify the source (s) of H+ ions
- Assume that although this is a weak acid, it’s still a much stronger acid than water
Write the ionization reaction and equilibrium constant equation
Construct your ICE table a. Initial acid concentration is given b. [H+ ]eq is determined by the pH
Calculate Ka
Check assumption (is Ka >> Kw?)
- Ex: A 0.050 mol/L solution of HC2H6NO2(aq) has a pH of 3.08. Calculate the Ka