Inflammation and cancer:biology to targeted therapies
Inflammation and cancer:biology to targeted therapies
Inflammation: cancer initiation:
- Chronic inflammation initiates tumour formation and creates genetic instability which leads to more mutations
- Inflammation and a single mutation is sufficient to drive tumour promotion
Tumour microenvironment:
- Necrotic cells are Dead cells
- Hypoxia is reduced oxygenation
- Immature blood vessels in tumour are prone to collapse
- When collapse tumour cells surrounding blood vessels will no longer be supplied with oxygen
- Tumour cell growth can outstrip blood supply causing hypoxia as blood is only supplied to cell which is close to blood vessel
- Hypoxia causes cells to switch on cytokines that cause angiogenesis
- Lymphatic vessels and blood vessels are routes in which a tumour cell can spread and travel to another environment in the body
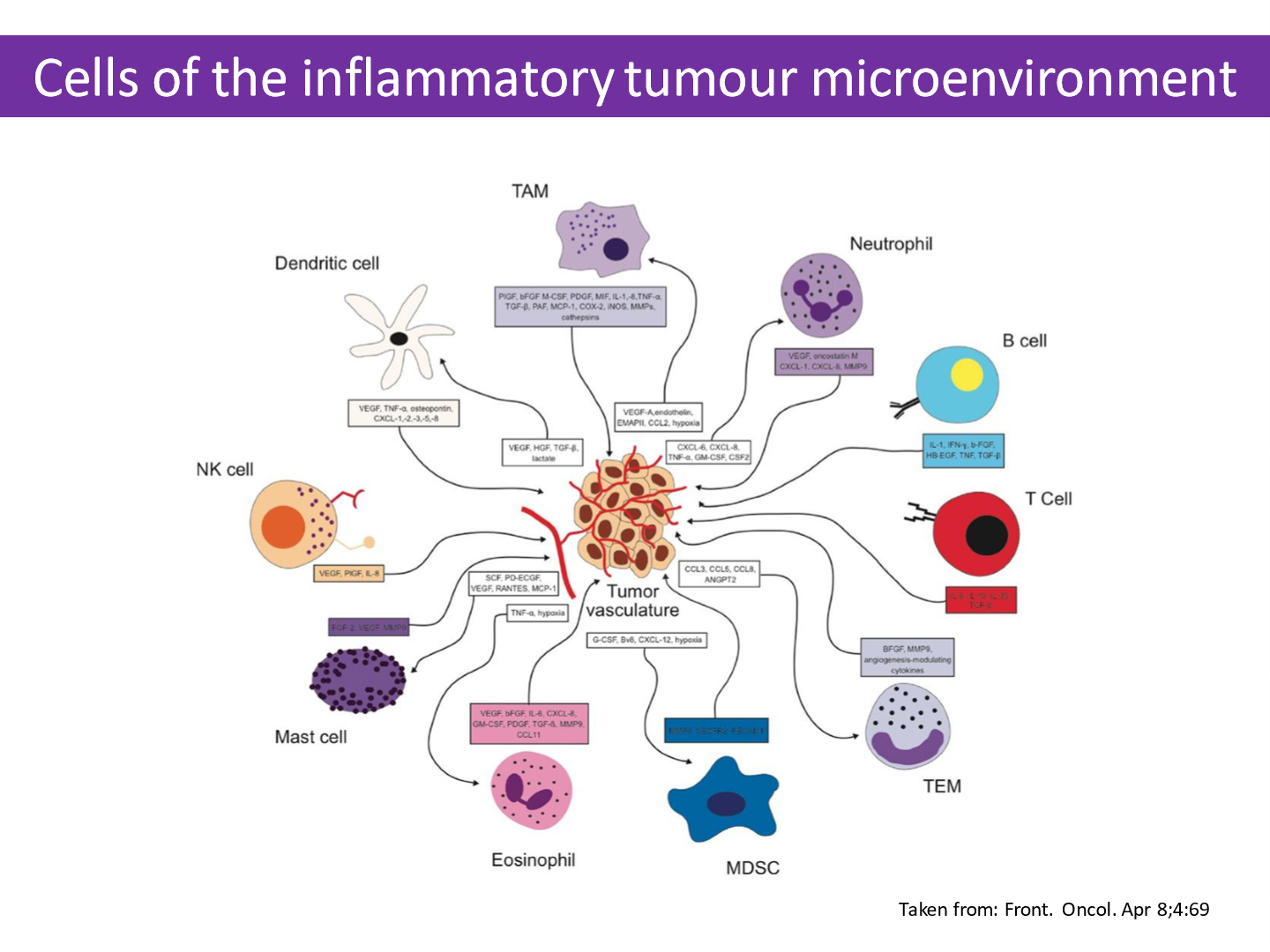
- Contributors to tumour microenvironment:
- Cancer cells
- Endothelial cells
- Fibroblasts
- Immune cells
- Secreted soluble growth factors
- Extra-cellular matrix
- Physiological and mechanical stress
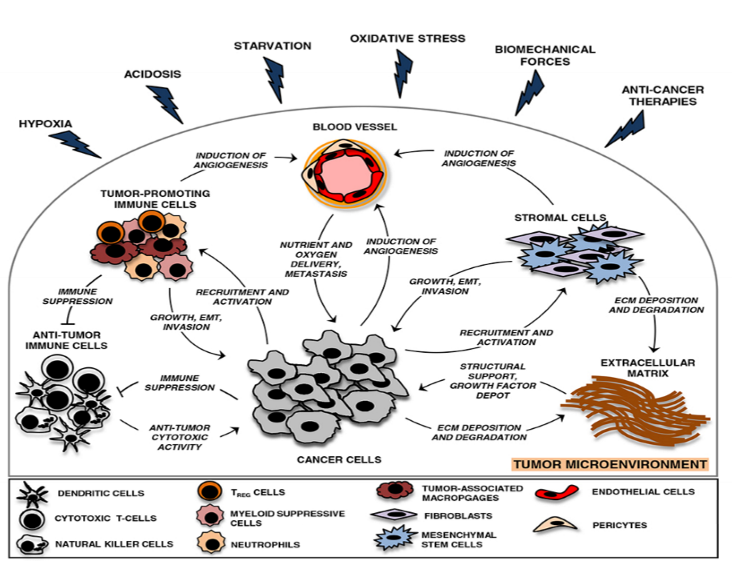
How hypoxia can benefit tumour growth:
- When oxygen concentration decline in tumours leads to hypoxia- directed secretion of cytokines and chemokines which recruit tumour promoting immune cells and supress antitumor immune responses
Immune surveillance:
- When tumours die they release antigens
- These antigens are then presented on surface of cancer cells
- T-cells are primed and activated
- T- cells migrate to site of tumour through blood vessels
- T cells then infiltrate tumour and recognise can cells
- After cancer cells recognised they are killed by T cells
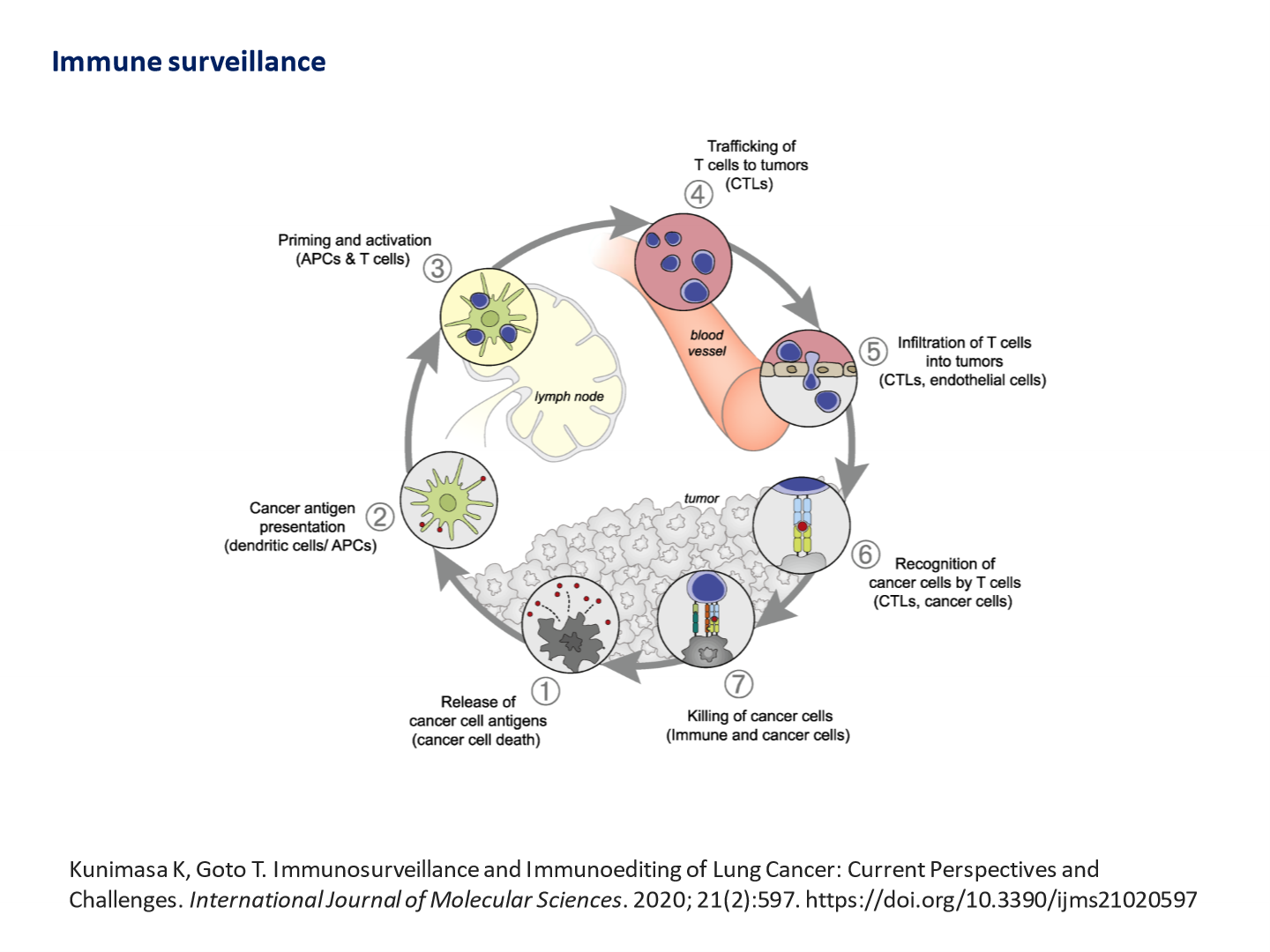
- Repression of autoimmunity enables cancer cell progression
Harnessing immune system to treat cancer - immunotherapy:
- Tumours aim to reprogram immune cells to become pro-tumorigenic
- Inhibiting pro-tumour inflammation:
- Identify key targets which produce immunosuppressive molecules
- Block cancer signalling pathways which drive pro-tumour inflammation
- Targeting STAT3 aims to prevent changes in cancer and immune cells which result in immuno-suppressive tumour microenvironment
- Promoting anti-tumour inflammation
- PD1 receptor is expressed on surface of activated T-cells
- Ligand (PD-L1) is expressed on dendritic cells or macrophages
- They are co-inhibitory immune checkpoint proteins
- Cancer cells express PD-L1 to evade the immune response
- Radio + chemotherapy lead to upregulated PDL-1 expression
- PD1 + PD-L1 can be targeted using immune checkpoint inhibitors
- Checkpoint inhibitor blocks the inhibition effect causing the T-cell to be switched on as recognising antigen on tumour cell which results in lysis of the tumour cell